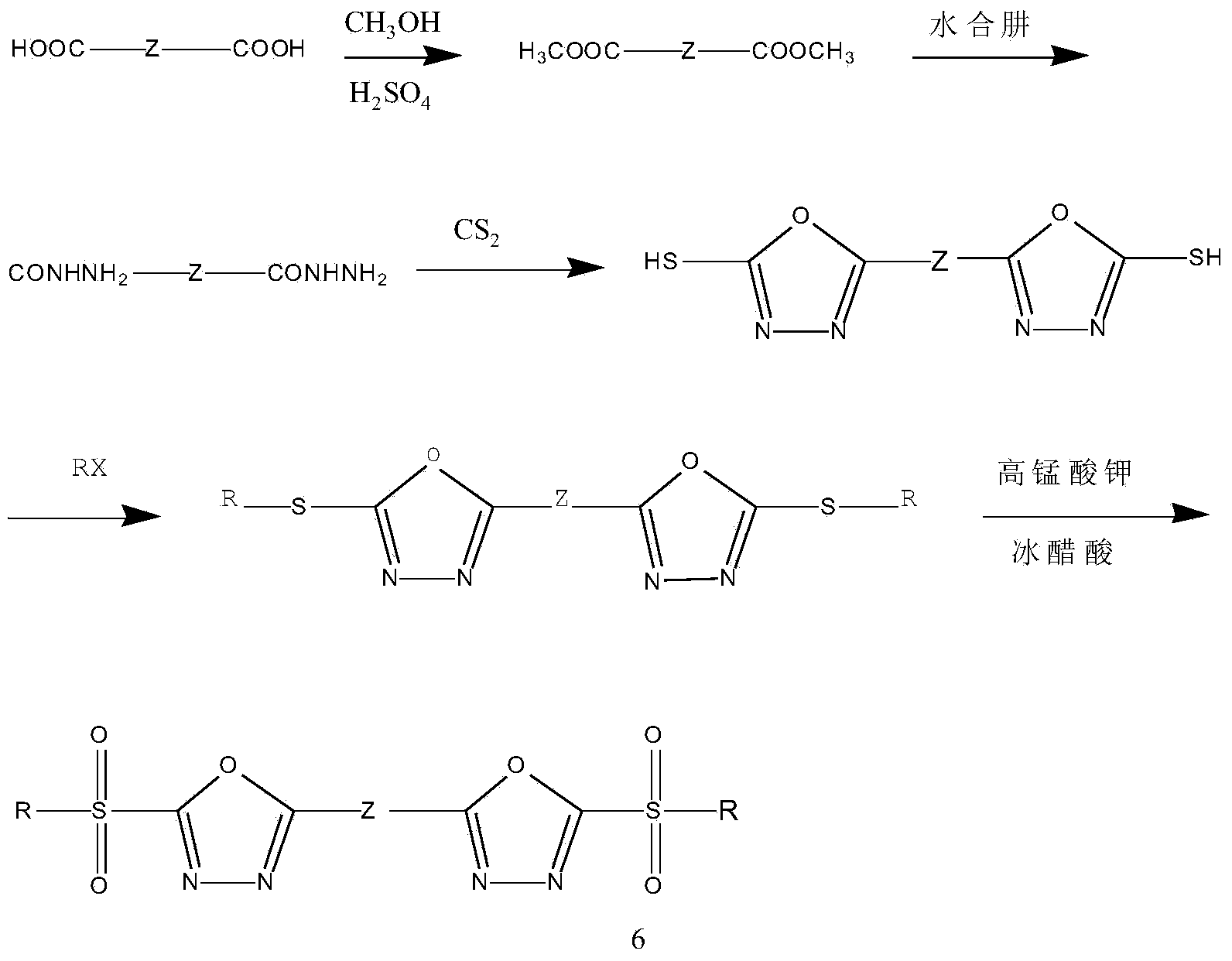
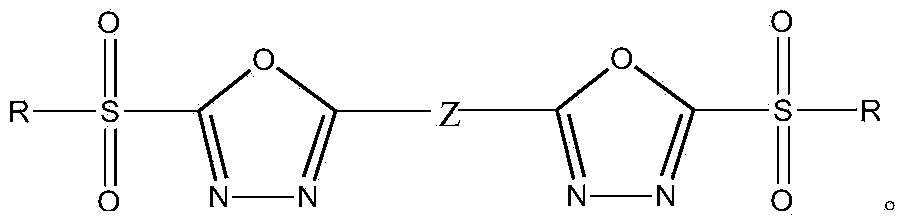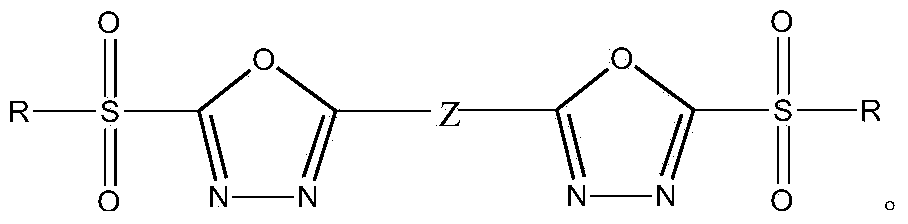Heterocyclic compound containing bis(2-substituted sulfonyl-1,3,4-oxadiazole-5-radical) and application thereof
A technology for heterocyclic compounds and oxadiazoles, which is applied in the fields of chemical industry and pesticides, can solve problems such as unreported synthesis of bisoxadiazoles, and achieve the effects of good inhibitory activity, good control effect and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of bis(2-hydrocarbylsulfonyl-1,3,4-oxadiazol-5-yl)benzene and its substitutes
[0060] (1) Synthesis of substituted dimethyl phthalate intermediate
[0061] Add 0.10 mol of substituted phthalic acid indicated by the general formula in a round bottom flask, dissolve in 150 mL of methanol, add 15 mL of concentrated sulfuric acid, and reflux for 24 hours. Pour into 500mL of water, separate, wash with water, and dry to obtain dimethyl substituted phthalate.
[0062] (2) Synthesis of Substituted Phthalohydrazide Intermediates
[0063]Add 0.07 mol of substituted dimethyl phthalate and 150 mL of methanol into a round bottom flask, add 100 mL of hydrazine hydrate, reflux for 24 hours, cool to room temperature, let stand for 5 hours, filter, wash with water, and dry to obtain substituted phthalic hydrazide.
[0064] (3) Synthesis of substituted 1,3-bis(2-mercapto-1,3,4-oxadiazol-5-yl)benzene intermediate
[0065] Add 0.03mol of substituted phthalohydrazide to the ...
Embodiment 2
[0085] Preparation of bis(2-hydrocarbylsulfonyl-1,3,4-oxadiazol-5-yl)biphenyl and its substitutes
[0086] (1) Synthesis of substituted dimethyl diphenylcarboxylate
[0087] Add 0.10 mol g of the substituted biphenyl dicarboxylic acid indicated in the general reaction formula to a round bottom flask, dissolve it in 150 mL of methanol, add 15 mL of concentrated sulfuric acid, and reflux for 24 hours. Pour into 500mL of water, separate, wash with water, and dry to obtain dimethyl substituted biphenyl dicarboxylate.
[0088] (2) Synthesis of substituted biphenyl dicarboxylic hydrazides
[0089] Add 0.07 mol of dimethyl substituted biphenyl dicarboxylate and 150 mL of methanol into a round bottom flask, add 100 mL of hydrazine hydrate, reflux for 24 hours, cool to room temperature, let stand for 5 hours, filter, wash with water, and dry to obtain substituted biphenyl dicarboxylic acid hydrazide .
[0090] (3) Synthesis of substituted bis(2-mercapto-1,3,4-oxadiazol-5-yl)biphenyl...
Embodiment 3
[0104] Example 3: Preparation of 2,6-bis(2-methylsulfonyl-1,3,4-oxadiazol-5-yl)-naphthalene
[0105] (1) Synthesis of dimethyl 2,6-naphthalene dicarboxylate
[0106] Add 20 g of 2,6-naphthalene dicarboxylic acid to a round bottom flask, dissolve it in 150 mL of methanol, add 15 mL of concentrated sulfuric acid, and reflux for 24 hours. Pour into 500mL of water, precipitate for 2 hours, filter, wash with water, and dry to obtain 20g of dimethyl 2,6-naphthalene dicarboxylate, with a yield of 89%.
[0107] (2) Synthesis of 2,6-naphthalene dicarboxylic acid hydrazide
[0108] Add 15g of dimethyl 2,6-naphthalene dicarboxylate and 150mL of methanol into a round bottom flask, add 80mL of hydrazine hydrate, reflux for 24 hours, cool to room temperature, let stand for 5 hours, filter, wash with water, and dry to obtain 2,6-naphthalene dicarboxylate Formic hydrazide 12.5g, yield 83%.
[0109] (3) Synthesis of 2,6-bis(2-mercapto-1,3,4-oxadiazol-5-yl)naphthalene
[0110] Add 5 g of 2,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com