Preparation method and application of immortalized human cartilage endplate stem cell line
A stem cell line and immortalization technology, applied in the field of cell engineering, can solve the problems of poor vitality and difficulty in survival, and achieve the effect of strong osteogenic ability, strong osteogenic differentiation ability, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
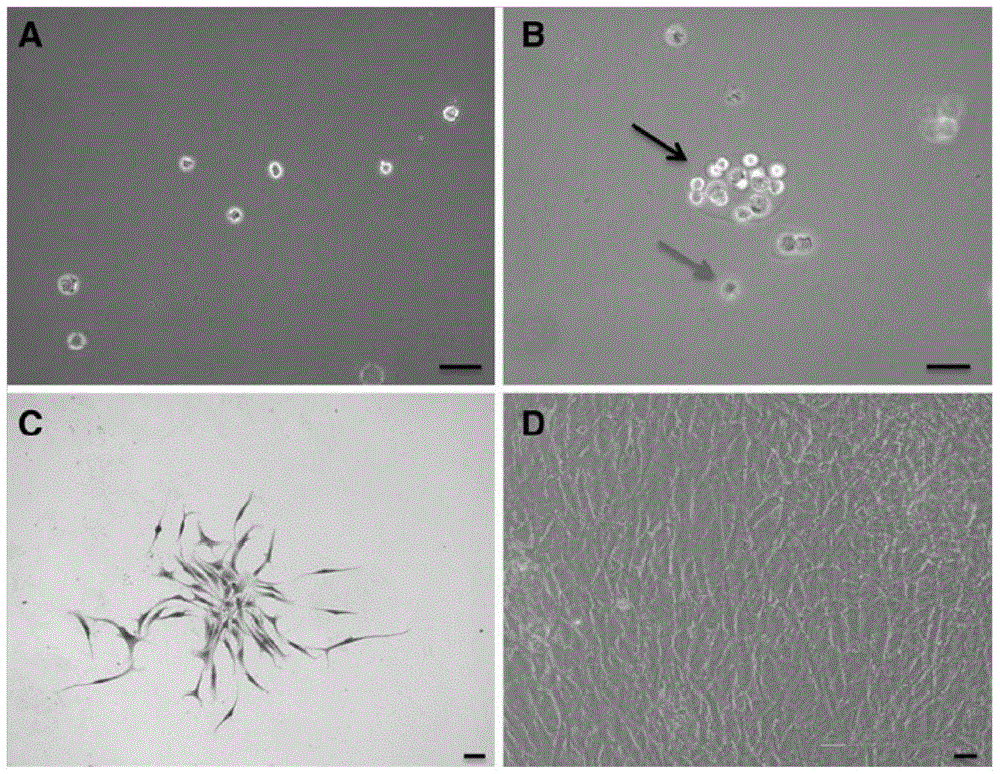
[0028] The acquisition, separation and identification of embodiment 1 CESCs
[0029] Inclusion criteria for cases from specimen sources: age younger than 65 and older than 30 years old with informed consent; no tuberculosis, hepatitis, tumor, diabetes and other infectious diseases and autoimmune diseases; cervical, thoracic and lumbar fusion surgery.
[0030] The cartilage endplate specimens discarded during the operation were separated with a dissecting microscope under sterile conditions, the cotton-like nucleus pulposus tissue and the white fibrous ring fibrous tissue were removed, and the adherent tissue was carefully scraped off with a sharp knife. The nucleus pulposus on the cartilage endplate, leaving the hyaline cartilage endplate, was confirmed by hematoxylin-eosin staining (Huang B, Liu LT, Li CQ, et al. Study to determine the presence of progenitor cells in the degenerated human cartilage endplates. Eur SpineJ, 2012, 21:613-622). The primary cells of cartilage endp...
Embodiment 2
[0033] Example 2 Construction of a lentiviral vector encoding the SV40T antigen and packaging of viral particles
[0034] Construction of a lentiviral vector encoding the SV40T antigen: The lentiviral vector plasmid pWPT-GFP (Shanghai Jikai Gene Chemical Technology Co., Ltd.) was double-digested with BamHI and SalⅠ enzymes from NEB Company to release GFP. The enzyme digestion conditions were 50 μl reaction system Add 1 μl of BamHI and SalⅠ, respectively, and incubate at 37°C for 30min. Finally, the digested product was subjected to 1% low-melting point agarose gel electrophoresis, and the carrier pWPT fragment was recovered with a gel recovery kit from Qiagen, Germany. The plasmid Plox-Ttag-iresTK (Shanghai Jikai Gene Chemical Technology Co., Ltd.) and the plasmid Plox-SV40-iresTK (Shanghai Jikai Gene Chemical Technology Co., Ltd.) were double-digested with SalⅠ and EcoRI, and the digestion conditions were 37°C for 30min , 65°C for 20min to inactivate the enzyme; then smooth ...
Embodiment 3
[0036] Example 3 Infection of Human Cartilage Endplate Stem Cells by Lentiviral Particles
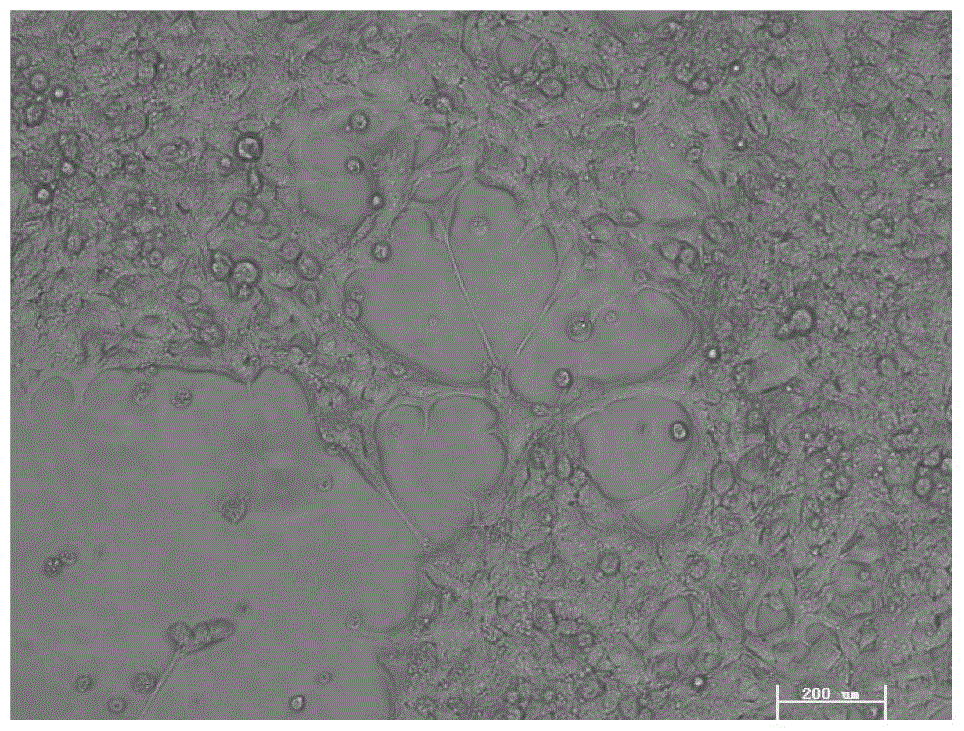
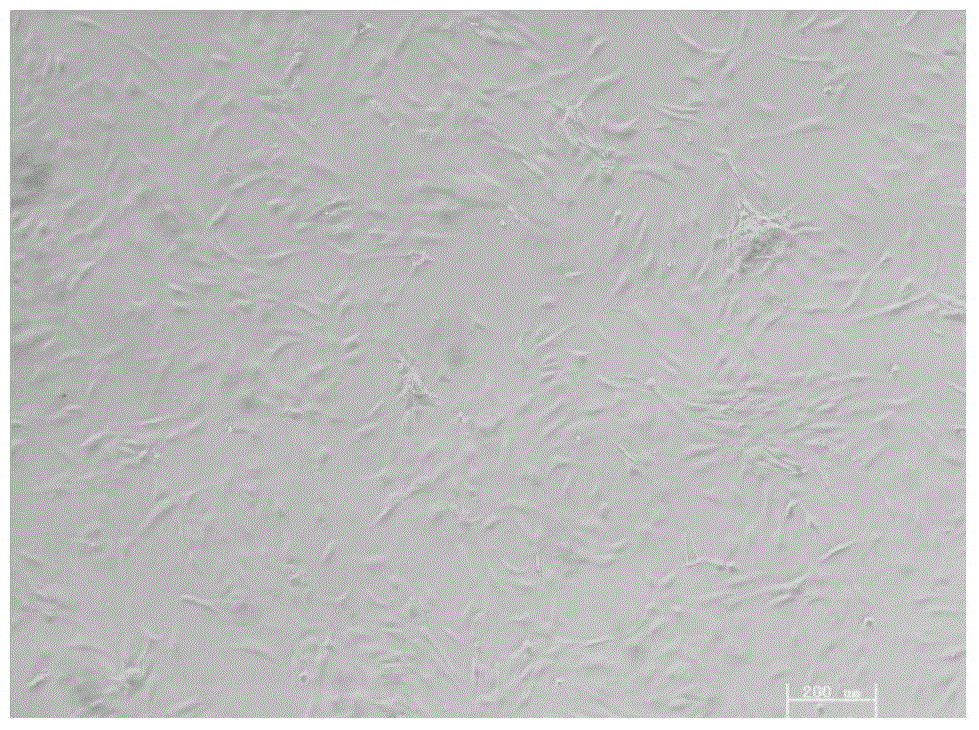
[0037] The CESCs cells passed to the third generation were planted in T25 cell culture flasks, and 2ml of lentiviral particles containing 4μg / ml polybrene were added, and the transfected cartilage endplate stem cells were as follows: image 3 shown. After 2 days of infection, apply 0.4mg / ml hygromycin B to select for 14 days to form cell colonies after selection, such as Figure 4 shown. The screened stem cell colonies were expanded and passed continuously for more than 30 passages to obtain the immortalized human degenerative cartilage endplate stem cell line iCESCs, which were cryopreserved for future use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com