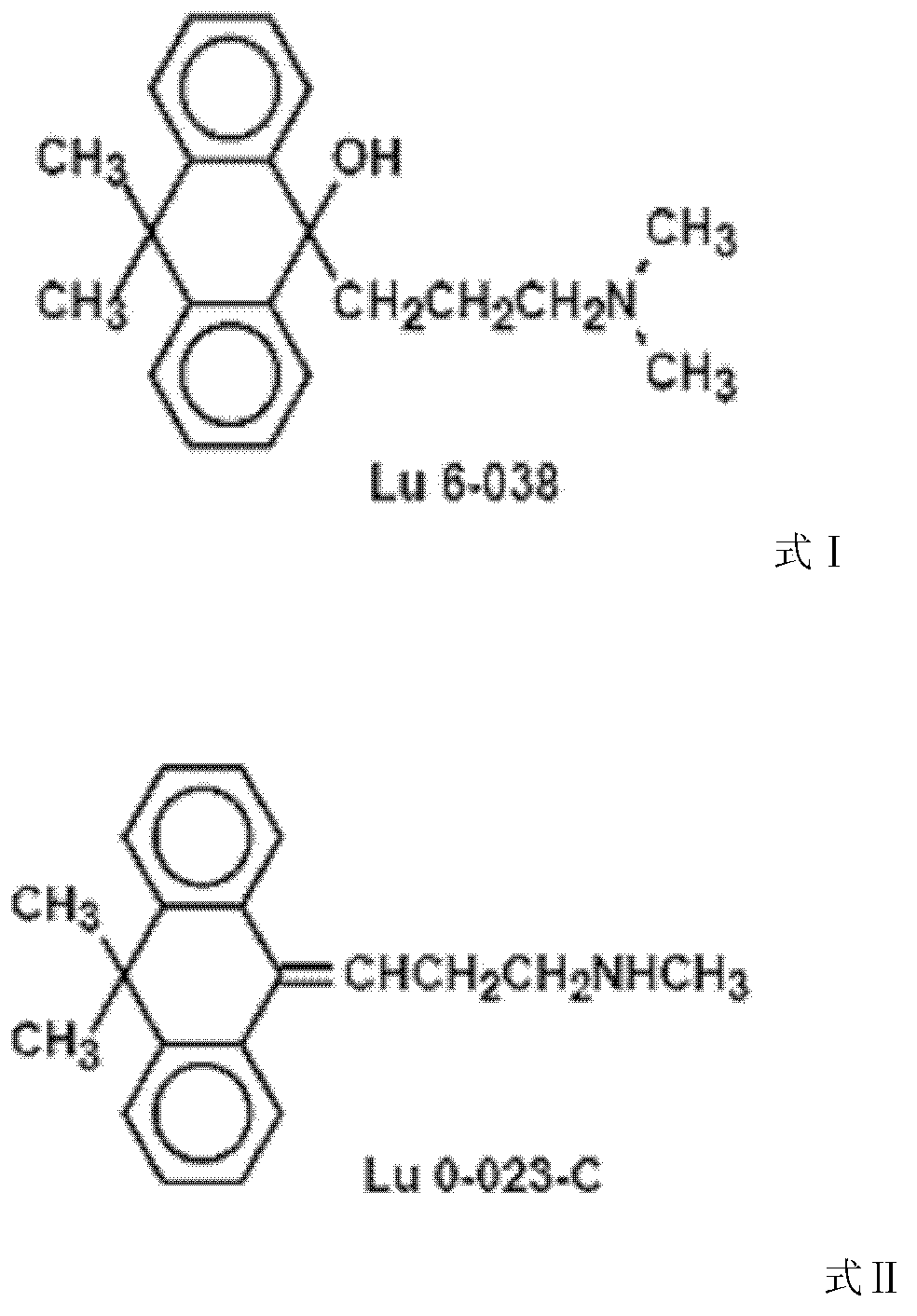
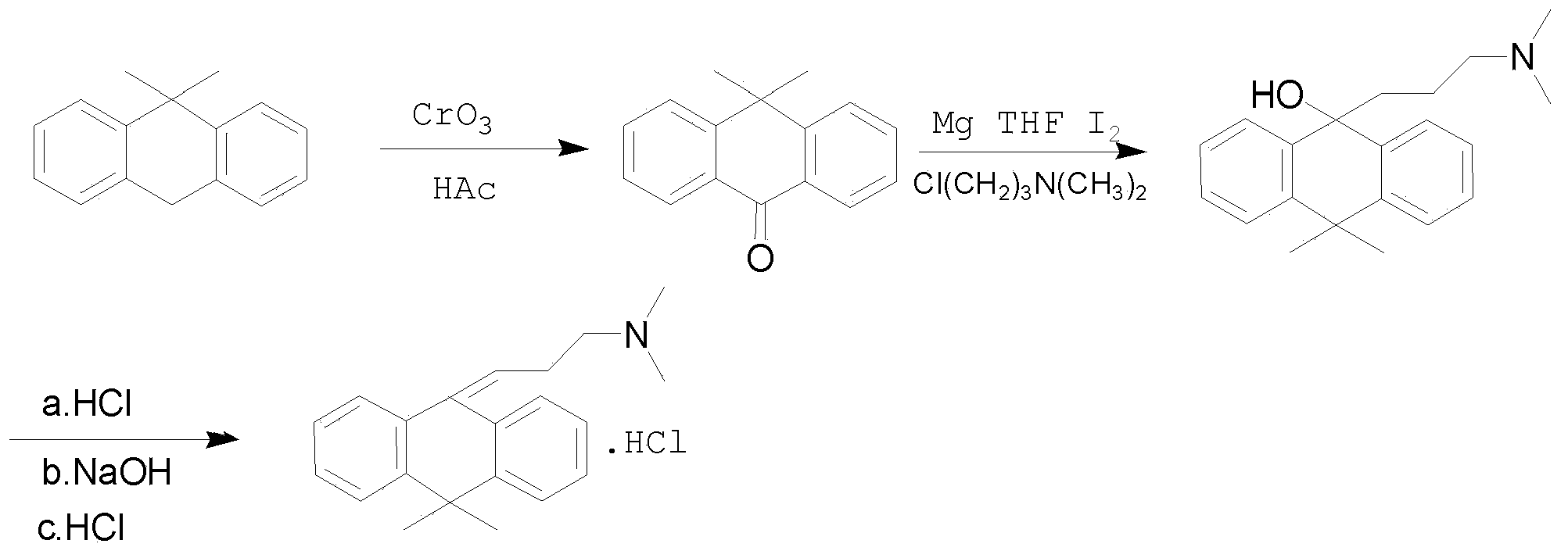
Melitracen pharmaceutical composition with high security
A kind of technology of melitracen and composition, applied in the field of melitracen pharmaceutical composition, can solve the problem of no antipsychotic effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation method of embodiment 1. compound Lu6-038
[0050] (1) Preparation of 10,10-dimethylanthrone
[0051] Add 13g (0.063mol) of 9,9-dimethyl-9,10-dihydroanthrone and 130mL of glacial acetic acid into the reaction flask, heat to 55~58°C, dissolve 10g (0.1mol) of chromium trioxide in 450mL Add glacial acetic acid solution (50%) dropwise to the reaction bottle, then react at 55~58°C for 4 hours, remove the solvent by rotary evaporation, add 130mL of water to the residue, stir and heat slightly to completely disperse the solid, Add 130 mL of water, stir for 10 min, filter, wash with water, extract the solid with dichloromethane, wash the organic layer with water, dry, and concentrate to dryness, and recrystallize the residue with ethanol-water (3:1) to obtain 10.2 g of white needle-like crystals, which are set aside .
[0052] (2) Preparation of 10,10-dimethyl-9γ-dimethylaminopropyl-9-anthracenol
[0053] Add 1.35g (0.056mol) of magnesium chips, 50mL of anhy...
Embodiment 2
[0054] Embodiment 2. Preparation of compound Lu0-023
[0055] Add 1.35g (0.056mol) of magnesium chips, 50mL of anhydrous tetrahydrofuran and 3 grains of iodine into the reaction flask, heat slightly, add 10.20g (0.084mol) of N-methyl-3-chloropropylamine, heat up and reflux for 2.5h, and cool to Room temperature; add 8.3g (0.038mol) of 10,10-dimethylanthrone to the above solution, stir at room temperature for 30min, add 45mL of water, separate the organic phase and the aqueous phase, extract with 35mL of dichloromethane, combine the organic phase, Dry over magnesium sulfate and concentrate to dryness to obtain 10.3 g of 3-[10,10-dimethyl-9(10H)-anthracene]-N-methylpropylamine, namely compound Lu0-023. Yield 85.3%, mp 82~85°.
Embodiment 3
[0057] The preparation method and synthetic process of melitracen hydrochloride are shown in figure 2 .
[0058] Add 10g (0.032mol) 10,10-dimethyl-9γ-dimethylaminopropyl-9-anthracenol, 23.5mL dichloromethane and 6.7mL concentrated hydrochloric acid into the reaction flask, heat to reflux for 2h, and cool to room temperature , extracted three times with water and dichloromethane, adjusted the pH of the aqueous layer to 8~9 with sodium hydroxide, extracted with dichloromethane, dried the dichloromethane layer with anhydrous sodium sulfate, concentrated and evaporated to dryness to obtain the free base of melitracen compound, it was dissolved in acetone, concentrated hydrochloric acid was added dropwise, and concentrated to dryness to obtain 7.3g melitracen. Yield 77.9%, mp 246~248°.
[0059] Elemental analysis (C 21 h 25 N·HCl)
[0060] Measured value % (theoretical value, %): C77.08 (76.92), H8.02 (7.99), N4.33 (4.27), C l 10.75 (10.81). MS-ESIm / z:292[M + H] + .
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap