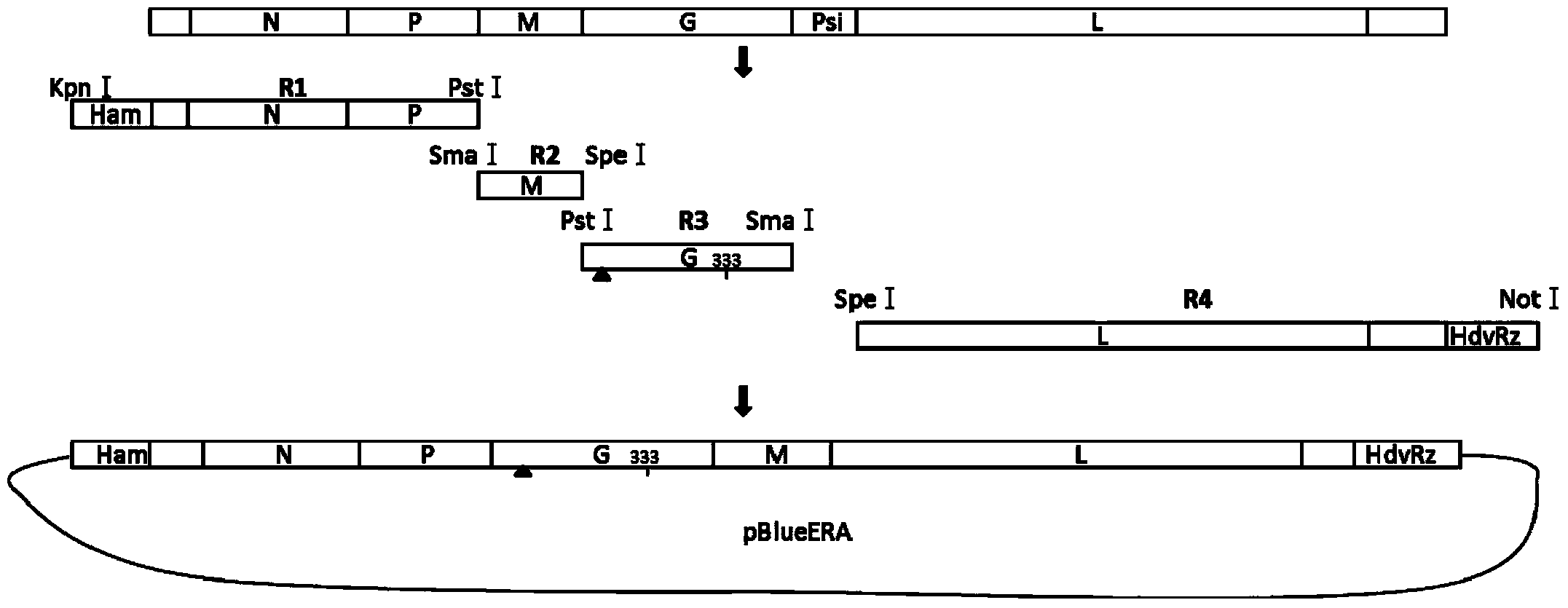
Rabies live vaccine and method for preparing rabies live vaccine for oral administration
A technology for rabies and live vaccines, applied in antiviral agents, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve problems such as difficulty in carrying out and easily harm medical personnel, and achieve the effect of improving efficiency and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] The preparation of embodiment 1 rabies virus antigen
[0091] (1) Suspension acclimatization of BHK-21 cells
[0092] Select the well-grown monolayer adherent cells of the BHK-21 cell clone, digest them with 0.01% trypsin-0.01% EDTA digestion solution, and suspend them to a final concentration of 2×10 5 / mL~5×10 5 / mL of cell fluid, the inoculum volume depends on the size of the tank and the amount of culture fluid. Suspension nutrient solution composition comprises: 88% MEM, 8% newborn bovine serum, 1% L-glutamine and antibiotic etc., then use (7.2%) NaHCO 2 Adjust the pH value to about 7.4, inoculate a 5L bioreactor, and cultivate at 37°C with 100rpm stirring. Follow this method for 60 passages, observe the cell morphology and count.
[0093] (2) Antigen preparation
[0094] Select the 13th generation of rabies ERAg3m strain adapted by BHK-21 cells, LD 50 for 10 -7.0 / ml, inoculated with 55-60 generations of suspension cells, the initial concentration of suspen...
Embodiment 2
[0095] The preparation of embodiment 2 microspheres
[0096] Dissolve 1g of chitosan in 1% acetic acid solution, and then add rabies virus antigen to the chitosan solution at a stirring speed of 200r / min, so that the final concentration of rabies virus antigen is 1×10 8.0 TCID 50 / ml, then slowly add sodium tripolyphosphate aqueous solution, mix evenly, centrifuge at 4 degrees Celsius and 15000-18000rpm and collect the precipitate, wash the precipitate with double distilled water and freeze-dry to obtain chitosan nanospheres , wherein the mass ratio of chitosan to sodium tripolyphosphate is (3:1) to (8:1).
Embodiment 3
[0097] The application of embodiment 3 rabies virus microspheres oral live vaccine
[0098] Inoculate 10 60-day-old stray dogs without rabies virus antibodies with live rabies virus microspheres orally, and set up 10 inactivated vaccination control dogs and 10 blank control dogs; 21 days after immunization, rabies virus street virus The above-mentioned dogs inoculated with rabies virus microspheres oral live vaccine and inactivated vaccine and blank control dogs were challenged with lethal dose. The specific results are shown in Table 1.
[0099] Table 1 rabies vaccine challenge protection results
[0100] group
[0101] Conclusion: 90% protection of dogs with live vaccine orally, 80% protection of dogs with inactivated vaccine, and blank control dogs all died without protection. Therefore, the challenge protection rate of the rabies virus microsphere oral live vaccine is significantly higher than that of the inactivated vaccine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com