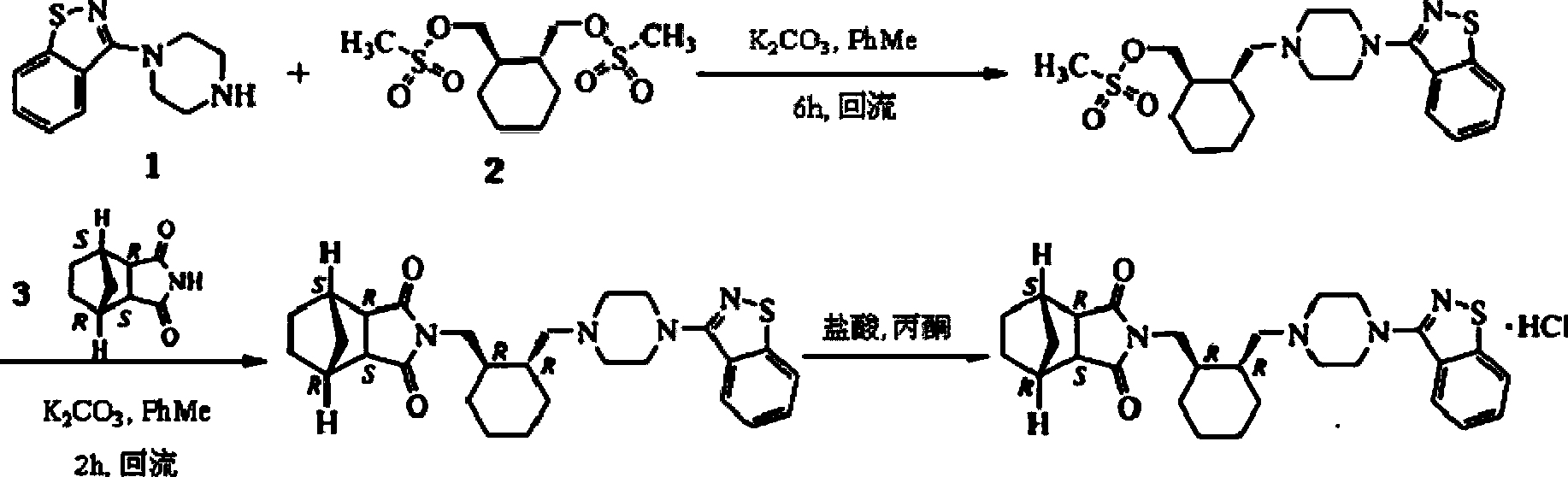
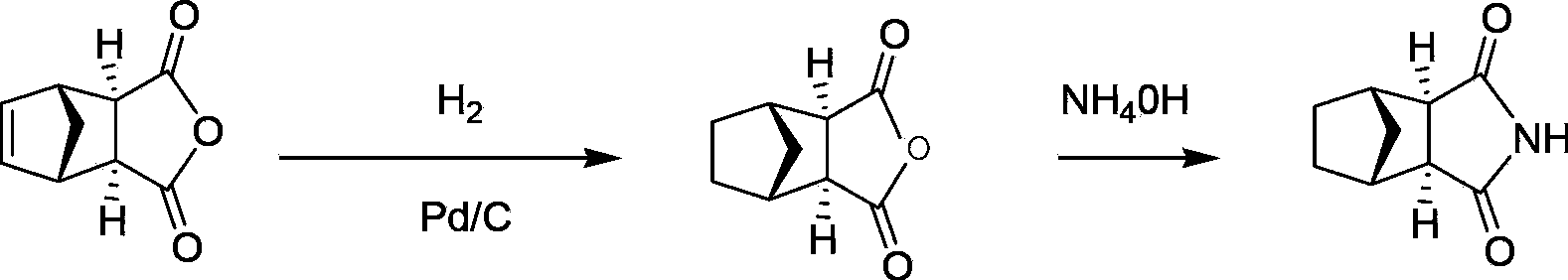
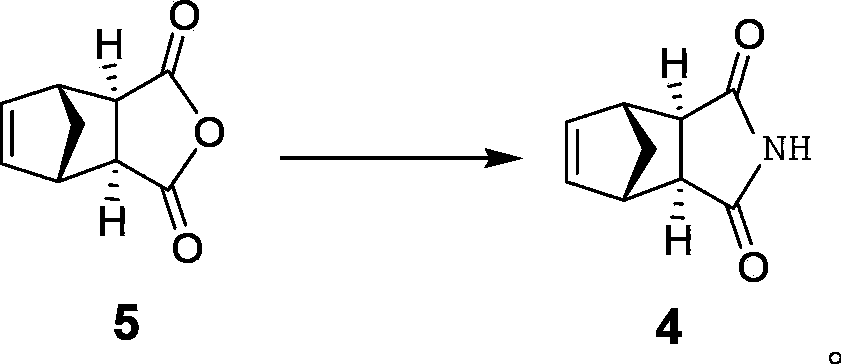
Preparation method of intermediate of lurasidone
A technology for lurasidone and intermediates, which is applied in the field of pharmaceutical synthesis, can solve the problems of harsh reaction conditions in the synthesis process, potential safety hazards, high temperature and high pressure, etc., and achieves the effects of less impurities, simple preparation process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of cis-5-norbornene-exo-2,3-dicarboximide (Compound 4)
[0033] Add 30ml of xylene, 20g of the compound cis-5-norbornene-exo-2,3-dicarboxylic anhydride, 14.6g of urea into a 250ml three-necked flask, heat to 135°C and stir for 6 hours, then cool down and distill off the solvent under reduced pressure. Recrystallize with 120ml of water, filter, and dry the filter cake at 70°C to obtain 17.2g of compound 4, yield: 86%, melting point: 160-162°C, 1H-NMR (CD 3 OD)δ: 1.38(m, 1H), 1.51(m, 1H), 2.71(d, J=1.6Hz, 2H), 3.16(m, 2H), 6.29(m, 2H), HPLC purity: 99.1%, Endo isomer product 0.3%, percentage is mass fraction.
Embodiment 2
[0035] Synthesis of cis-5-norbornene-exo-2,3-dicarboximide (Compound 4)
[0036] Add 30ml of N,N-dimethylformamide, 20g of the compound cis-5-norbornene-exo-2,3-dicarboxylic acid anhydride, and 15.3g of ammonium formate into a 250ml three-necked flask, heat to 140°C and stir for 7 hours , cooled and evaporated the solvent under reduced pressure, recrystallized with 120ml of water, filtered, and dried the filter cake at 70°C to obtain 16.6g of compound 4, yield: 83%, endo isomer product 0.25%, percentages are mass fractions.
Embodiment 3
[0038] Synthesis of cis-5-norbornene-exo-2,3-dicarboximide (Compound 4)
[0039] Add 30ml of N,N-dimethylformamide, 20g of compound cis-5-norbornene-exo-2,3-dicarboxylic acid anhydride, 14.6g of urea into a 250ml three-necked flask, heat to 135°C and stir for 6 hours, The solvent was evaporated under reduced pressure, recrystallized with 120ml of water, filtered, and the filter cake was dried at 70°C to obtain 17.4g of compound 4, yield: 87%, endo isomer product 0.28%, percentages are mass fractions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com