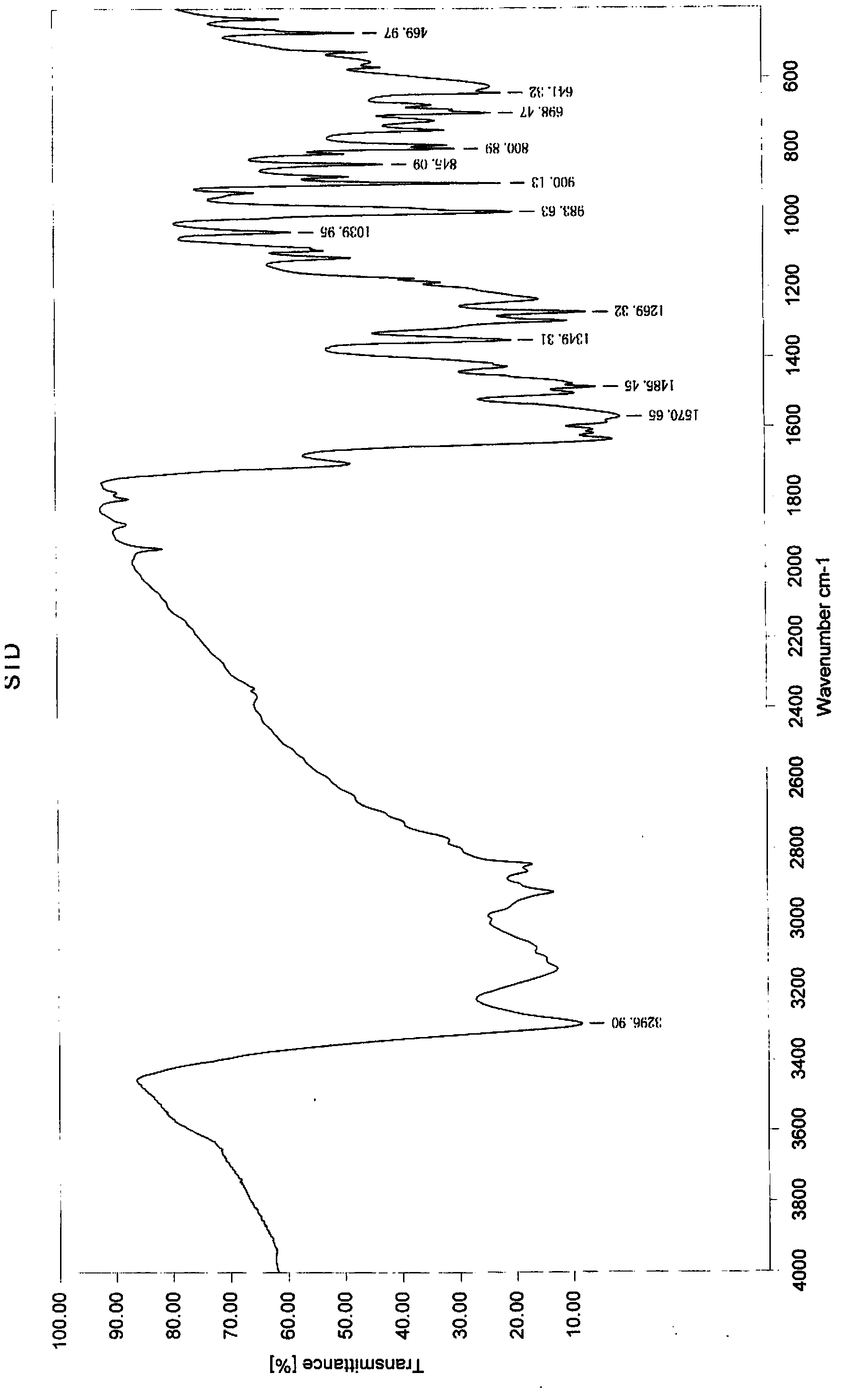
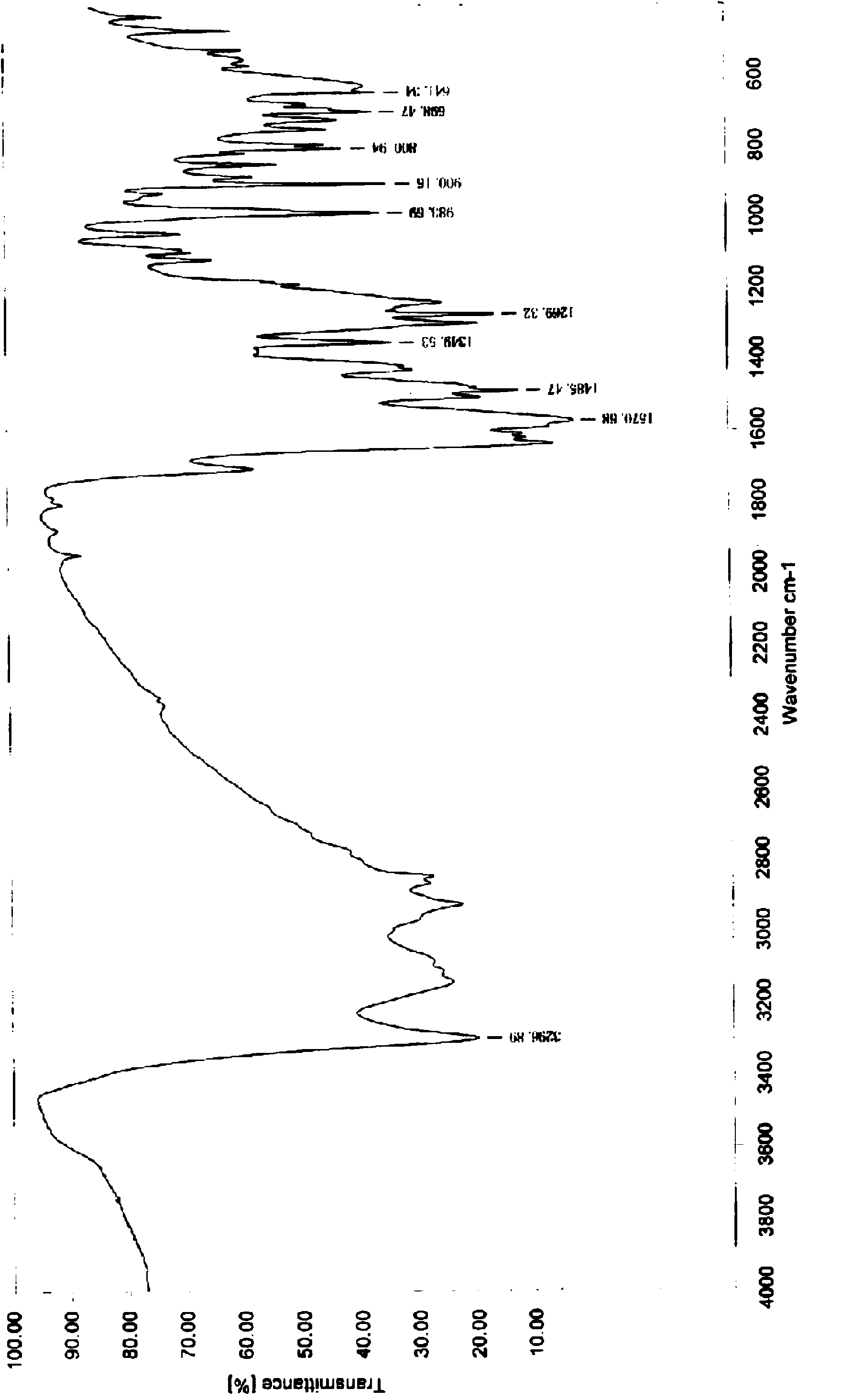
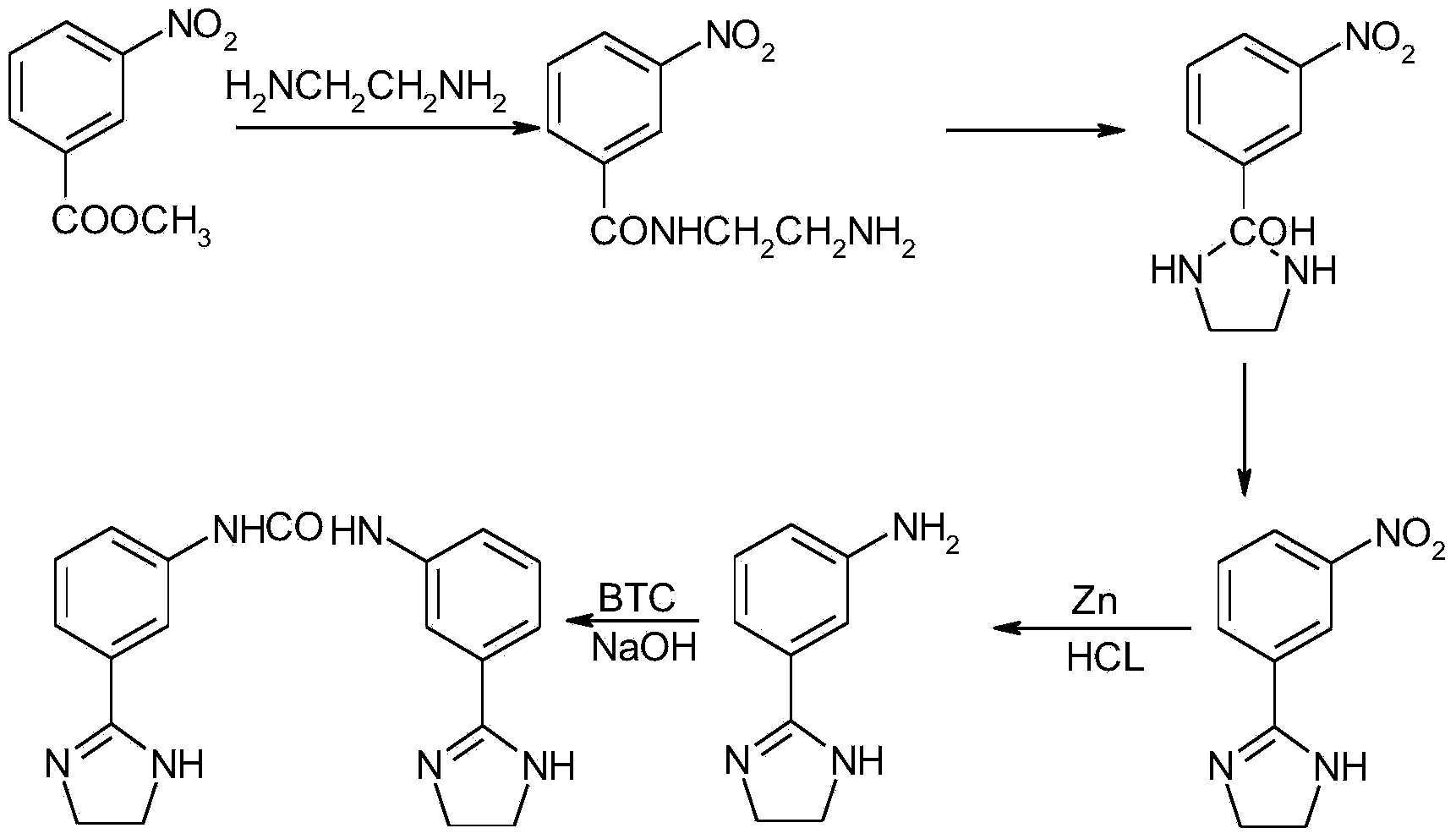
Preparation method of imidocarb
A technology of imidazobenzeneurea and nitroimidazoline, which is applied in the field of compound synthesis, can solve the problems of high energy consumption, long production cycle, and many wastes, and achieves the effects of mild reaction conditions, convenient operation and pollution avoidance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Dissolve 18.1 g of methyl m-nitrobenzoate, 6.0 g of ethylenediamine, and 0.16 g of sulfur in 110 ml of methanol, reflux at 65°C for 4 hours, evaporate most of the solvent under reduced pressure, and filter to obtain m-nitroimidazoline , and the molar yield was 95.0%.
[0024] Take 17.2g of the intermediate product m-nitroimidazoline, dissolve it in 90ml of 50% methanol, adjust the pH value to 4.0 with 5mol / L hydrochloric acid, and add 7.0g of zinc powder while stirring in four times at normal temperature within 2 hours, and the addition is completed. The stirring reaction was continued for 1 hour. After the reaction was finished, the zinc chloride was removed by filtration, most of the solvent was evaporated under reduced pressure, and the crystallized m-aminoimidazoline had a molar yield of 91.3%.
[0025] Then take 13.0g of m-aminoimidazoline and dissolve it in 65ml of tetrahydrofuran-water (1:5) solution, adjust the pH value to 8 with 0.1mol / L sodium hydroxide solut...
Embodiment 2
[0027] Dissolve 18.1 g of methyl m-nitrobenzoate, 9.0 g of ethylenediamine, and 0.16 g of sulfur in 110 ml of methanol, reflux at 65°C for 5 hours, evaporate most of the solvent under reduced pressure, and filter to obtain m-nitroimidazoline , and the molar yield was 95.1%.
[0028] Then take 17.2g of the intermediate product m-nitroimidazoline, dissolve it in 90ml of 50% methanol, adjust the pH value to 3.5 with 5mol / L hydrochloric acid, add 8.0g of zinc powder while stirring in four times at normal temperature within 2 hours, and complete the addition. The stirring reaction was continued for 2 hours. After the reaction was finished, zinc chloride was removed by filtration, most of the solvent was evaporated under reduced pressure, and the crystallized m-aminoimidazoline had a molar yield of 91.7%.
[0029] Dissolve 13.0 g of m-aminoimidazoline in 65 ml of tetrahydrofuran-water (1:5, V:V) solution, adjust the pH value to 10 with 0.1 mol / l sodium hydroxide solution, add 0.08 ...
Embodiment 3
[0031] Take 18.1g of methyl m-nitrobenzoate, 4.0g of ethylenediamine, and dissolve 0.32g of sulfur in 181ml of methanol. After reflux at 65°C for 3 hours, add 2.0g of ethylenediamine and reflux for 2 hours, and distill out under reduced pressure. After removing most of the solvent, m-nitroimidazoline was obtained by filtration with a molar yield of 96.4%.
[0032] Then take 17.2g of the intermediate product m-nitroimidazoline, dissolve it in 90ml of 50% methanol, add 5mol / L hydrochloric acid to adjust the pH value to 3.5, add 8.8g of zinc powder while stirring in four times at normal temperature within 2 hours, and complete the addition The stirring reaction was continued for 2 hours. After the reaction was finished, the zinc chloride was removed by filtration, most of the solvent was evaporated under reduced pressure, and the crystallized m-aminoimidazoline had a molar yield of 92.0%.
[0033] Dissolve 13.0 g of m-aminoimidazoline in 65 ml of tetrahydrofuran-water (1:6, V:V)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com