Absorbent for recycling sulfur dioxide from industrial waste gas and recycling method
A technology for sulfur dioxide and industrial waste gas, applied in chemical instruments and methods, separation methods, and separation of dispersed particles, etc., can solve the problems of small absorption capacity, high regeneration energy consumption, and high solvent price, and achieve large absorption capacity and low regeneration energy consumption , The effect of low solvent price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
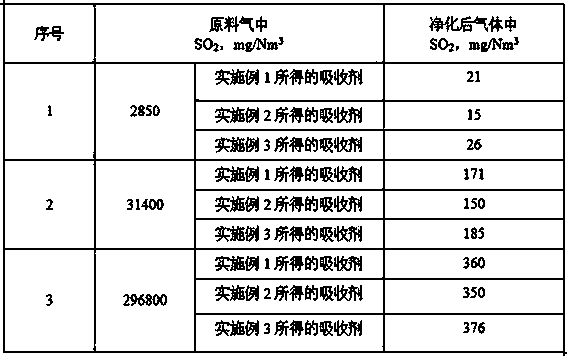
[0031] In the reaction kettle equipped with a thermometer, agitator, feeder, and cooler, add 100 mol of piperazine and 250 mol of water in sequence. After stirring evenly, replace the air in the reaction kettle with nitrogen and maintain the nitrogen flow. Add 125 mol of ethylene oxide dropwise, the dropwise addition is completed in 1 hour, and then react for 3 hours, and control the reaction temperature to 80°C, the pressure during the reaction is 0.2MPa, then add 20g of boric acid to the reactant, and react for 0.5 hours to obtain organic amine Salt. The organic amine salt is dissolved in water to make an organic amine salt aqueous solution with a concentration of 20% by mass to obtain an absorbent.
Embodiment 2
[0033] In the reaction kettle equipped with a thermometer, agitator, feeder, and cooler, add 100 mol of piperazine and 200 mol of water in sequence. After stirring evenly, replace the air in the reaction kettle with nitrogen and maintain the nitrogen flow. 120 mol of ethylene oxide was added dropwise, the reaction temperature was controlled to be 60°C, the pressure during the reaction was 0.1MPa, the dropwise addition was completed in 1 hour, and the reaction was continued for 2 hours, then 18g of boric acid was added to the reactant, and the reaction was carried out for 1.5 hours to obtain organic amine salt. The organic amine amine salt is dissolved in water to prepare an organic amine salt aqueous solution with a concentration of 4% by mass to obtain an absorbent.
Embodiment 3
[0035] In the reaction kettle equipped with a thermometer, agitator, feeder, and cooler, add 100 mol of piperazine and 400 mol of water in sequence. After stirring evenly, replace the air in the reaction kettle with nitrogen and maintain the nitrogen flow. 150 mol of ethylene oxide was added dropwise, the reaction temperature was controlled to be 100°C, the pressure during the reaction was 0.4MPa, the dropwise addition was completed in 1.5 hours, and the reaction was continued for 3.5 hours, then 20 g of boric acid was added to the reactant, and the reaction was carried out for 3 hours to obtain organic amine salt. The organic amine amine salt is dissolved in water to prepare an organic amine salt aqueous solution with a concentration of 35% by mass to obtain an absorbent.
[0036] The specific absorption method is;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com