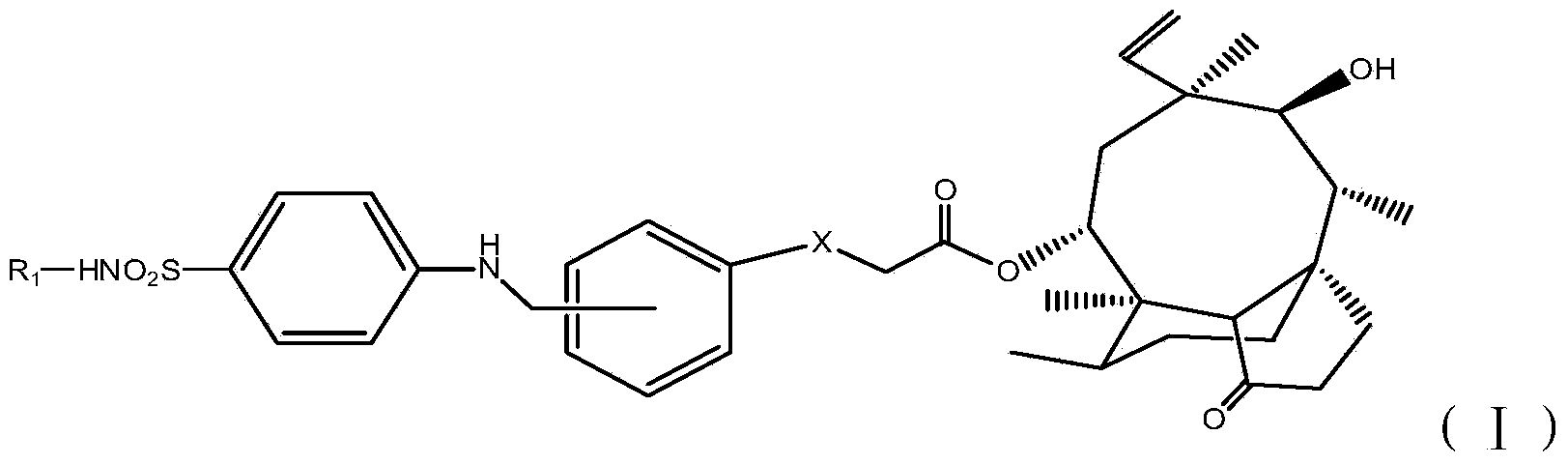
Pleuromutilin derivatives with antibacterial activity as well as preparation method and application thereof
A technology of pleuromutilin and antibacterial activity, applied in the direction of organic active ingredients, medical preparations containing active ingredients, antibacterial drugs, etc., can solve the problem of the generation of drug-resistant strains, the screening and discovery of new drug molecules, and the time-consuming, human and financial issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
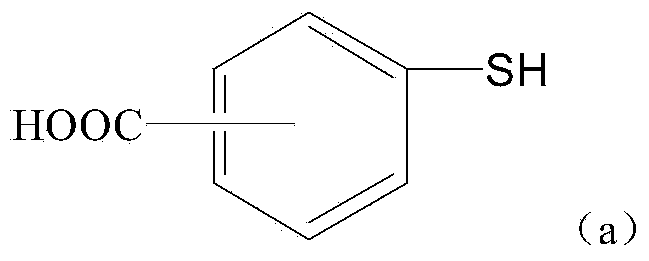
[0065] Synthesis of 4-mercaptobenzyl alcohol [formula (1)]
[0066] 4gLiAlH 4 Added to 50ml tetrahydrofuran to form a suspension, N 2 For protection, dissolve 8.5 g of 4-mercaptobenzoic acid into 100 ml of tetrahydrofuran, and slowly add it dropwise to the above suspension under ice-cooling. After the addition, continue to stir for 30 minutes, then raise the temperature to reflux, and stir for 16 hours. After the reaction, add 40ml ethyl acetate and 50ml15%H2O respectively. 2 SO 4 solution, filtered, the aqueous phase was extracted 3 times with 50ml ethyl acetate, the organic phases were combined, washed with saturated brine, anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain 7.5 g of light yellow or colorless liquid. Purified by silica gel column chromatography (ethyl acetate:petroleum ether=75:25) to obtain 7.1 g of white solid with a yield of 93% and a melting point of 51-53°C. MS-ESI (M+1): 141.2. The H NMR spectrum results are as follows:
[...
Embodiment 2
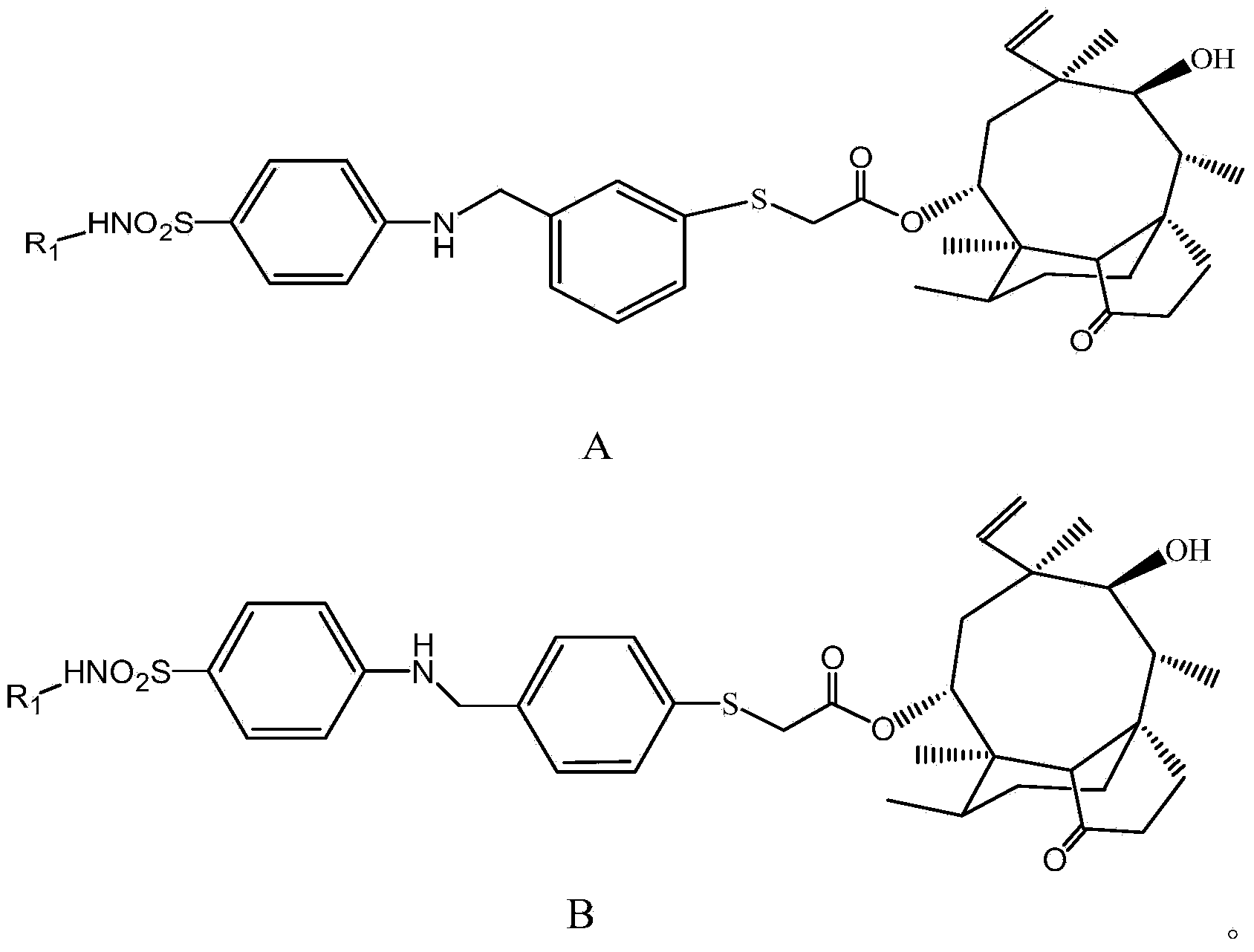
[0069] Synthesis of (4-hydroxymethyl-phenylmercapto)-acetyl-14-oxo-miaolin [formula (3)]
[0070] Add 11.0g of pleuromutilin formula (b) and 5.5g of p-toluenesulfonyl chloride to 50ml of ethyl acetate in sequence, wash in an ice bath, and adjust the pH value to strong with 20ml of 1mol / L potassium hydroxide solution Alkaline, heat up to 35°C, stir vigorously for 2.5 hours, and monitor the end of the reaction by thin-layer chromatography. After the reaction was complete, the organic solvent was distilled off under reduced pressure, and the residue was diluted 10 times with water, and the white solid was washed out, filtered with suction, washed with water until neutral, dried, and recrystallized from acetone to obtain 14.8 g of a white solid. The yield is 92%, MS-ESI (M+1): 533.1.
[0071] Dissolve 1.5g of sodium methoxide in 250ml of methanol, cool down to 15°C, add 4.8g of the product 4-mercaptobenzyl alcohol formula (1) of Example 1, and 17.6g of the product of step 1) succ...
Embodiment 3
[0074] Synthesis of (4-chloromethyl-phenylmercapto)-acetyl-14-oxo-miaolin [formula (5)]
[0075] 5g of the product formula (3) of Example 2 was dissolved in 100ml of dichloromethane, then 1.5g of triethylamine and 0.75ml of DMF were added, the temperature was lowered to 0°C, and 2.1g of SOCl was added dropwise under vigorous stirring 2 After adding 10 ml of dichloromethane solution, the temperature was naturally raised to room temperature, and stirring was continued for 1.5 h. After the reaction was completed, the reaction solution was poured into 80ml of ice water, extracted with ethyl acetate, separated, and the organic phase was washed with water until neutral, anhydrous Na 2 SO 4 Dry, concentrate under reduced pressure, and separate by silica gel column chromatography to obtain 5.8 g of a solid of formula (5). Yield 70.5%, melting point: 121.1-123.8 MS-ESI (M+1): 519.5. The proton nuclear magnetic resonance spectrum results are as follows:
[0076] 1 HNMR(DMSO)δ: 7.70...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com