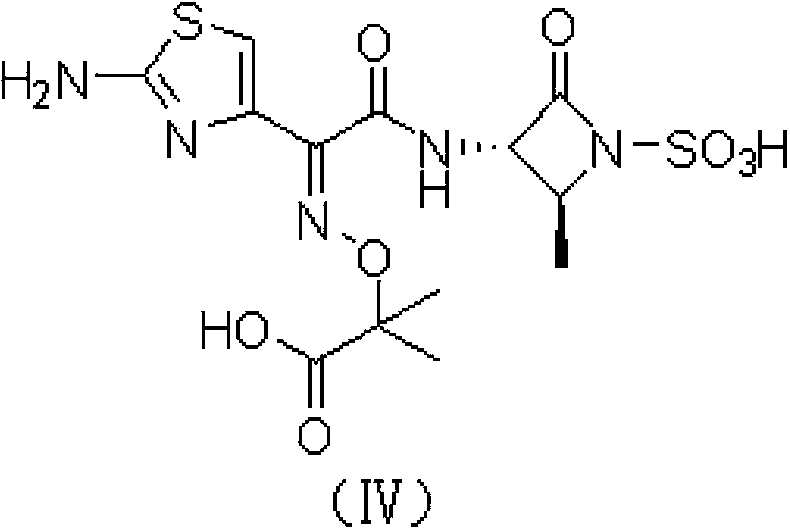
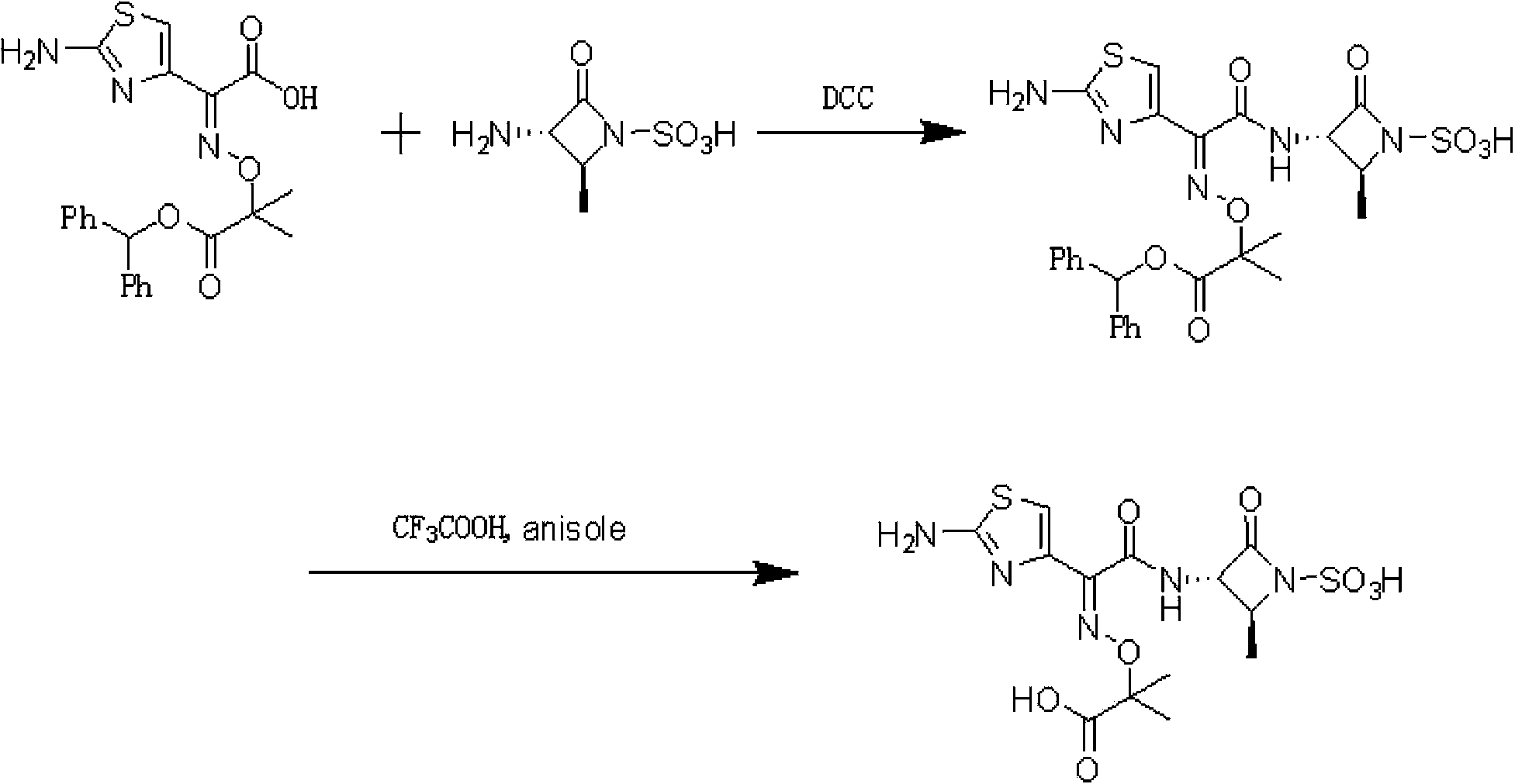
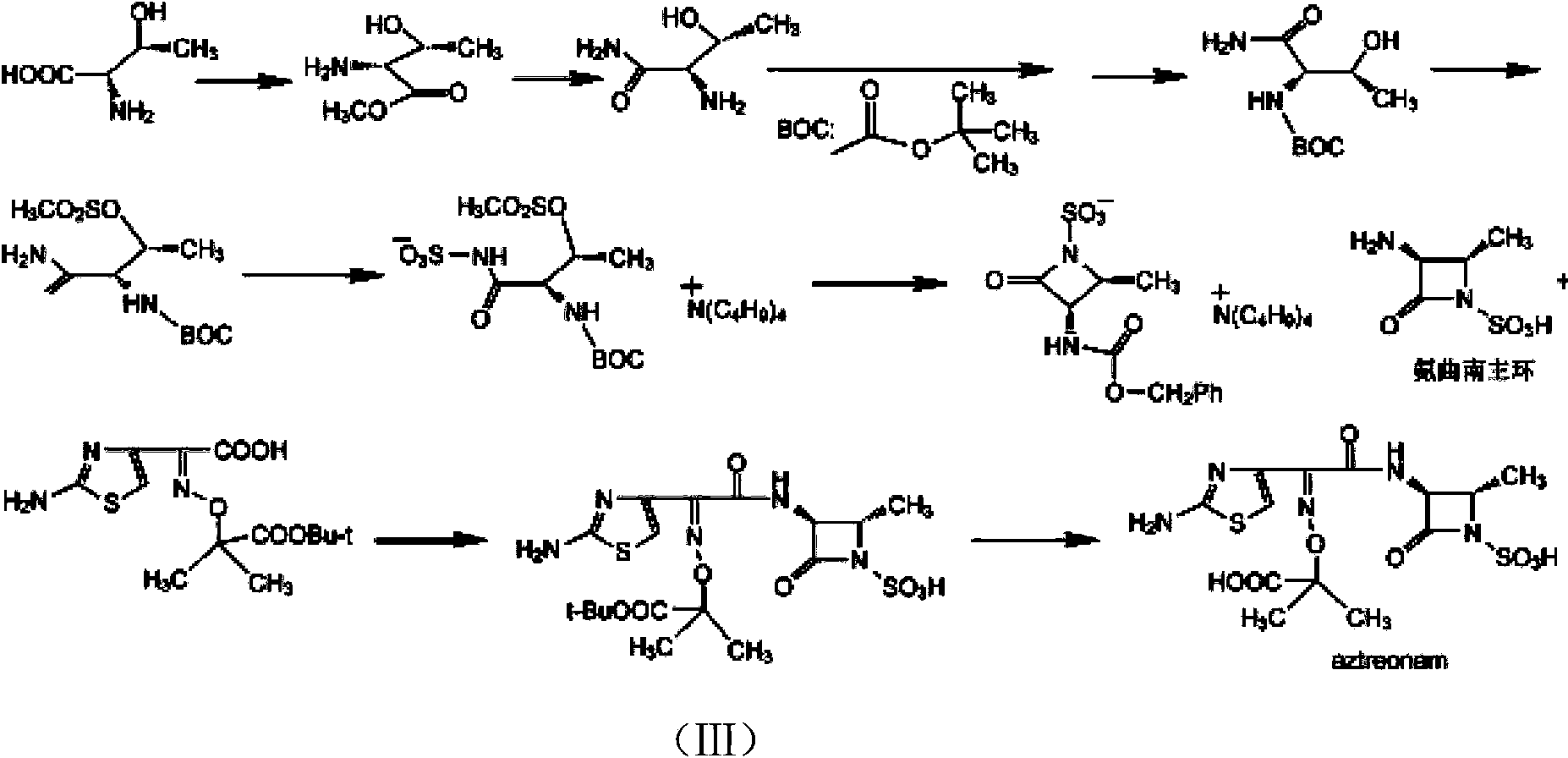
Aztreonam preparation method
A technology of aztreonam and amino, which is applied in the field of preparation of aztreonam compounds, can solve the problems of unfavorable industrial production, expensive raw materials, cumbersome steps, etc., and achieve the effects of stable source of raw materials, high purity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0037] Example 1 [2S-[2α,3β(Z)]]-2-[[[1-(2-amino-4-thiazolyl)-2-[(2-methyl-4-oxo-1- Preparation of tert-butyl sulfo-3-azetidinyl)amino]-2-oxoethylene]amino]oxo]-2-methylpropanoate
[0038] Mix 5 g of (S)-3-amino-2-oxo-1-azetidinesulfonic acid with (Z)-2-amino-a-[(1-tert-butoxycarbonyl-1-methylethoxy 14.61 g of benzothiazolethiol imino-4-thiazolyl acetate was put into the reaction flask, dissolved in 500 mL of acetone, and 4.3 mL of triethylamine was added at room temperature, stirred for 2.5 hours, cooled to 0°C, and added dropwise Trifluoroacetic acid, adjust the pH value to 4.5, then stir for 1.5 hours, filter, and dry the filter cake to obtain [3S-[3α(Z),4β]]-3-[[(2-Amino-4-thiazolyl)- [(1-tert-butoxycarbonyl-1-methylethoxy)imino]-acetyl]amino]-4-methyl-2-oxo-1-azetidinesulfonic acid 11g, product The rate is 79%.
Embodiment 2
[0039] The preparation of embodiment 2 aztreonam
[0040] [3S-[3α(Z),4β]]-3-[[(2-amino-4-thiazolyl)-[(1-tert-butoxycarbonyl-1-methylethoxy)imino] -Acetyl]amino]-4-methyl-2-oxo-1-azetidinesulfonic acid 5g was dissolved in 20mL of formic acid, added 30mL of anisole, cooled to 0°C, slowly added dropwise 2mol of dilute hydrochloric acid / L15mL, raised to room temperature after completion, stirred for 2 hours, then added ethyl acetate, continued to stir for 0.5 hours, filtered, washed the filter cake with ethyl acetate, dried to obtain 4 g of aztreonam crude product, yield 90%.
Embodiment 3
[0041] The refining of embodiment 3 aztreonam
[0042] Add 5g of crude product aztreonam into 5mL of deionized water, heat and stir to dissolve, add activated carbon for adsorption, filter, cool off, add 45mL of ethanol, cool to 5-10°C, stir, precipitate solid, filter, wash with absolute ethanol, Dried to obtain 4.5g of aztreonam compound refined product, yield 90%, HPLC purity 99.5%,
[0043] Chromatographic conditions:
[0044] Column: C 18 Column, 250×4.6mm, 5μm
[0045] Mobile phase: use 0.05mol / L potassium dihydrogen phosphate solution (adjust pH value to 3.0 with phosphoric acid)-acetonitrile (90:10) as mobile phase A, use 0.05mol / L potassium dihydrogen phosphate solution (adjust pH value with phosphoric acid) to 3.0) - acetonitrile (60:40) as mobile phase B; elute isocratically with mobile phase A first, and immediately perform linear gradient elution as shown in the table below after the aztreonam peak is eluted.
[0046]
[0047] Detection wavelength: 270nm
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com