Monodisperse laser-responsive photoinducedly-movable one-dimensional organic semiconductor microbelt, and preparation method and application thereof
An organic semiconductor, photo-induced mobility technology, applied in organic chemistry, nanotechnology for sensing, nanotechnology, etc., can solve the problems of high cost, electricity is not a direct energy source, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Four monomers of perylene anhydride-containing peryleneimide derivatives having the same substituents at both ends having the following molecular formula were prepared.
[0063]
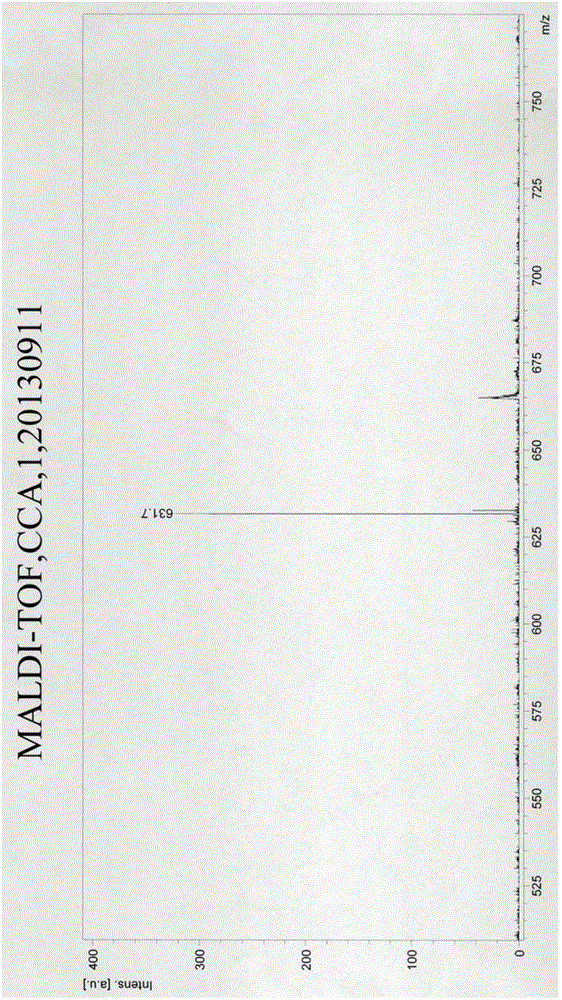
[0064] (1) Take 50 mg of perylene-3,4,9,10-tetracarboxylic dianhydride raw material, add 8 grams of imidazole and 250 microliters of 3-methoxybenzylamine to it, at a temperature of 130 ° C Carry out the reaction for 3 hours to obtain a reaction solution, then add 15 milliliters of concentrated hydrochloric acid with a mass concentration of 36% to the reaction solution and stir overnight, take out the product and wash it with water until the pH is neutral and then dry it to obtain 3-methoxy at both ends. Peryleneimide derivatives containing perylene anhydride substituted with benzyl groups; mass spectrum data of monomer 1 of this perylene imide derivative containing perylene anhydride substituted with 3-methoxybenzyl groups at both ends Such as figure 1 shown; or
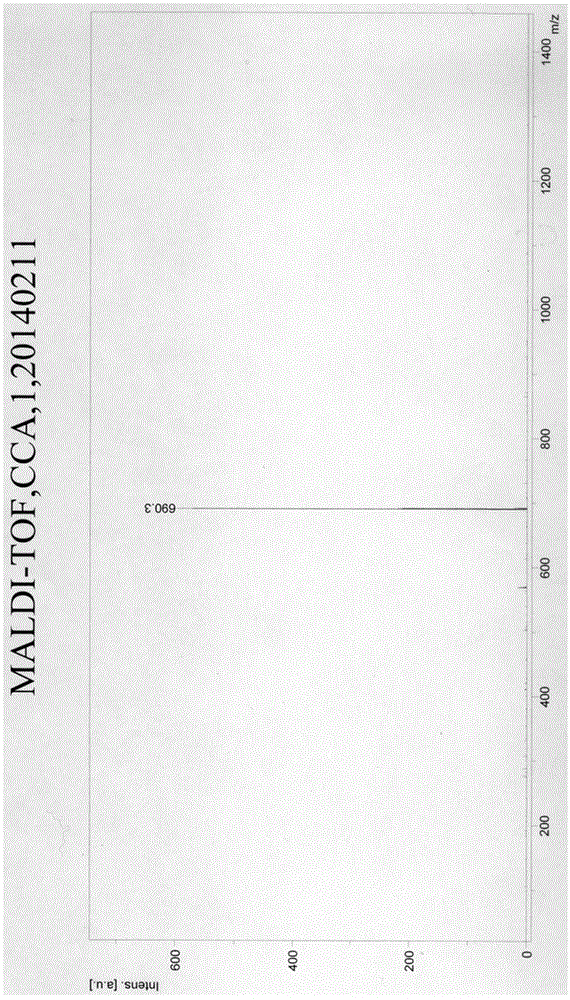
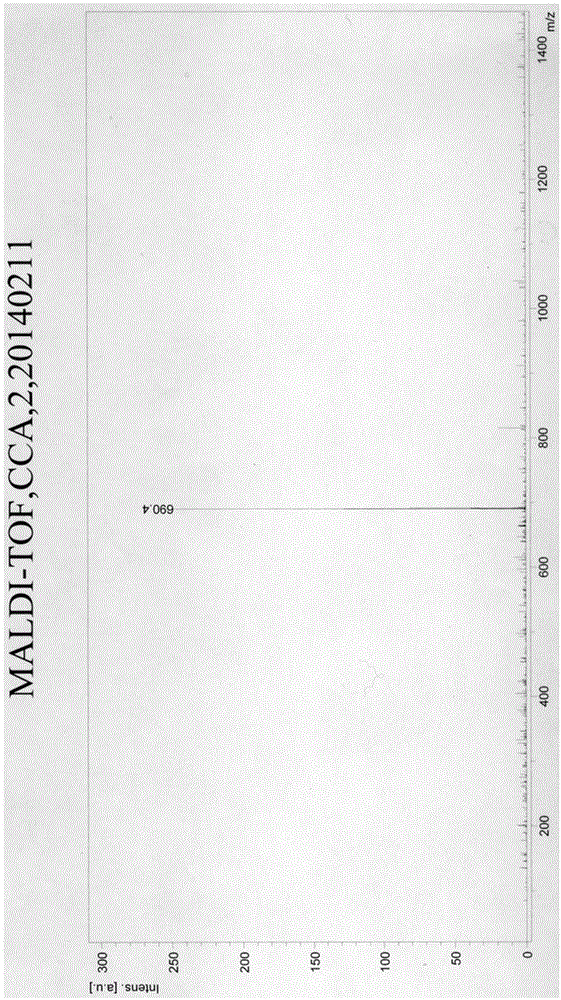
[0065] Take 50 mg of per...
Embodiment 2
[0071] The suspensions of four kinds of one-dimensional organic semiconductor microribbons containing multiple monodisperse laser-responsive photo-induced movement obtained in Example 1 were dripped on silicon wafers respectively, and placed in a desiccator to remove ethanol; After drying, the four kinds of monodisperse laser-responsive photoinduced movement one-dimensional organic semiconductor microribbons were respectively placed in a Leica ion sputtering instrument, and platinum particles with a particle size of 10 nm were loaded on the one-dimensional organic semiconductor micron ribbons. The surface of the belt is then placed into a field emission scanning electron microscope to observe the morphology, the results of the SEM image are as follows Figure 6-9 shown; among them, Figure 6 middle Figure 6 a shows the monodisperse laser-responsive photoinduced movement of multiple perylene imide derivatives (monomer 1) substituted with 3-methoxybenzyl groups at both ends. ...
Embodiment 3
[0073] Take a piece of clean glass and paste a layer of scotch tape (Scotch Tape) on it to prepare a hydrophobic glass substrate; or
[0074] Take a clean piece of glass, wash it with acetone, put it into the mixed solution of hydrogen peroxide and concentrated sulfuric acid (volume ratio is 3:1), soak it for 2 hours, take it out, wash it with acetone; then put the cleaned glass piece into Soak in trichloro(octadecyl)silane-toluene mixed solution (volume ratio 1:100) for 5 hours, take it out, wash it with acetone, and prepare a hydrophobic glass substrate;
[0075] The monodisperse laser-responsive photoinduced movement of the one-dimensional organic semiconductor prepared in Example 1 is composed of peryleneimide derivatives containing perylene anhydride substituted by 3-methoxybenzyl at both ends. The suspension of micron belts is dropped on the surface of the above-mentioned hydrophobic glass substrate, blown dry with nitrogen, and then use a laser with a wavelength of 488nm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com