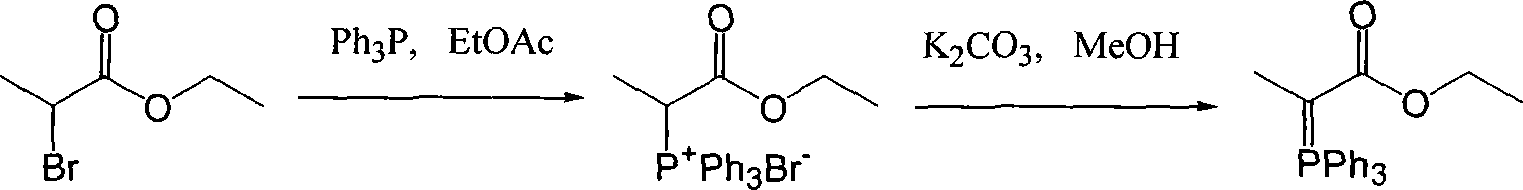
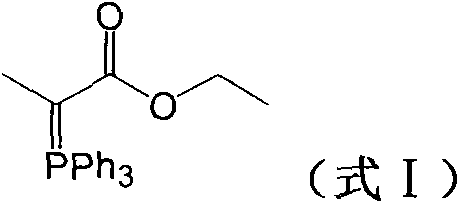
Preparation method of (carbethoxyethylidene)triphenylphosphorane
A technology of ethoxyformyl ethylene triphenylphosphine and triphenyl phosphine, which is applied in the field of preparation of ethoxyformyl ethylene triphenylphosphine, can solve the problem of pungent smell in the operating space and the increase of solvent Recovering costs, threatening human health and other issues, achieving the effects of shortening the production cycle, achieving continuity and high safety, and facilitating industrialized continuous production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 The preparation method of ethoxy formyl ethylene triphenylphosphine of the present invention
[0024] Add 131g of triphenylphosphine and 100g of ethyl 2-bromopropionate into a 1000mL three-neck flask, then add 200mL of toluene and 500ml of water, and stir at 80°C for 12h. After the reaction, cool to 50°C, separate the water layer, wash the water layer with 50ml of toluene, cool down to room temperature, add 10% sodium hydroxide solution dropwise to adjust the pH to 8.0-8.5, continue to stir for 1h, and filter to obtain the filter cake Dry at 50° C. to obtain 172 g of ethoxyformyl ethylene triphenylphosphine as a solid, with a yield of 95%.
[0025] The δ( 1 HNMR, DMSO-d 6 ): 7.40-7.80(m, 15H), 3.88(q, 0.92H, J=6.8Hz), 3.53(q, 1.18H, J=6.8Hz), 1.49(d, 3H, J=13.9Hz), 1.4 (t, 1.28H, J=6.8Hz), 0.38(t, 1.72H, J=6.8Hz) ppm. MS: 363 (MH + ).
Embodiment 2
[0026] Example 2 The preparation method of ethoxy formyl ethylene triphenylphosphine of the present invention
[0027] Add 131g of triphenylphosphine and 100g of ethyl 2-bromopropionate into a 1000mL three-neck flask, then add 200mL of toluene and 500ml of water, and stir at 80°C for 12h. After the reaction, cool to 50°C, separate the water layer, wash with 50ml of toluene, cool down to room temperature, add 10% potassium hydroxide solution dropwise to adjust the pH to 8.0-8.5, and continue stirring for 1h. After filtering, the resulting filter cake was dried at 50° C. to obtain 175 g of ethoxyformyl ethylene triphenylphosphine as a solid, with a yield of 96%.
[0028] The δ( 1 HNMR, DMSO-d 6 ): 7.40-7.80(m, 15H), 3.88(q, 0.92H, J=6.8Hz), 3.53(q, 1.18H, J=6.8Hz), 1.49(d, 3H, J=13.9Hz), 1.4 (t, 1.28H, J=6.8Hz), 0.38(t, 1.72H, J=6.8Hz) ppm. MS: 363 (MH + ).
Embodiment 3
[0029] Example 3 The preparation method of ethoxy formyl ethylene triphenylphosphine of the present invention
[0030] Add 131g of triphenylphosphine and 100g of ethyl 2-bromopropionate into a 1000mL three-neck flask, add 200mL of toluene and 500ml of water, and stir at 80°C for 12h. After the reaction, cool to 50°C, separate the water layer, wash with 50ml of toluene, cool down to room temperature, slowly add 20% sodium carbonate solution dropwise to adjust pH=8.0-8.5, continue to stir for 1h, filter, and the obtained filter cake is at 50°C Drying at a lower temperature yielded 160 g of ethoxyformyl ethylene triphenylphosphine as a solid, with a yield of 88%.
[0031] The δ(1HNMR, DMSO-d 6 ): 7.40-7.80(m, 15H), 3.88(q, 0.92H, J=6.8Hz), 3.53(q, 1.18H, J=6.8Hz), 1.49(d, 3H, J=13.9Hz), 1.4 (t, 1.28H, J=6.8Hz), 0.38(t, 1.72H, J=6.8Hz) ppm. MS: 363 (MH + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com