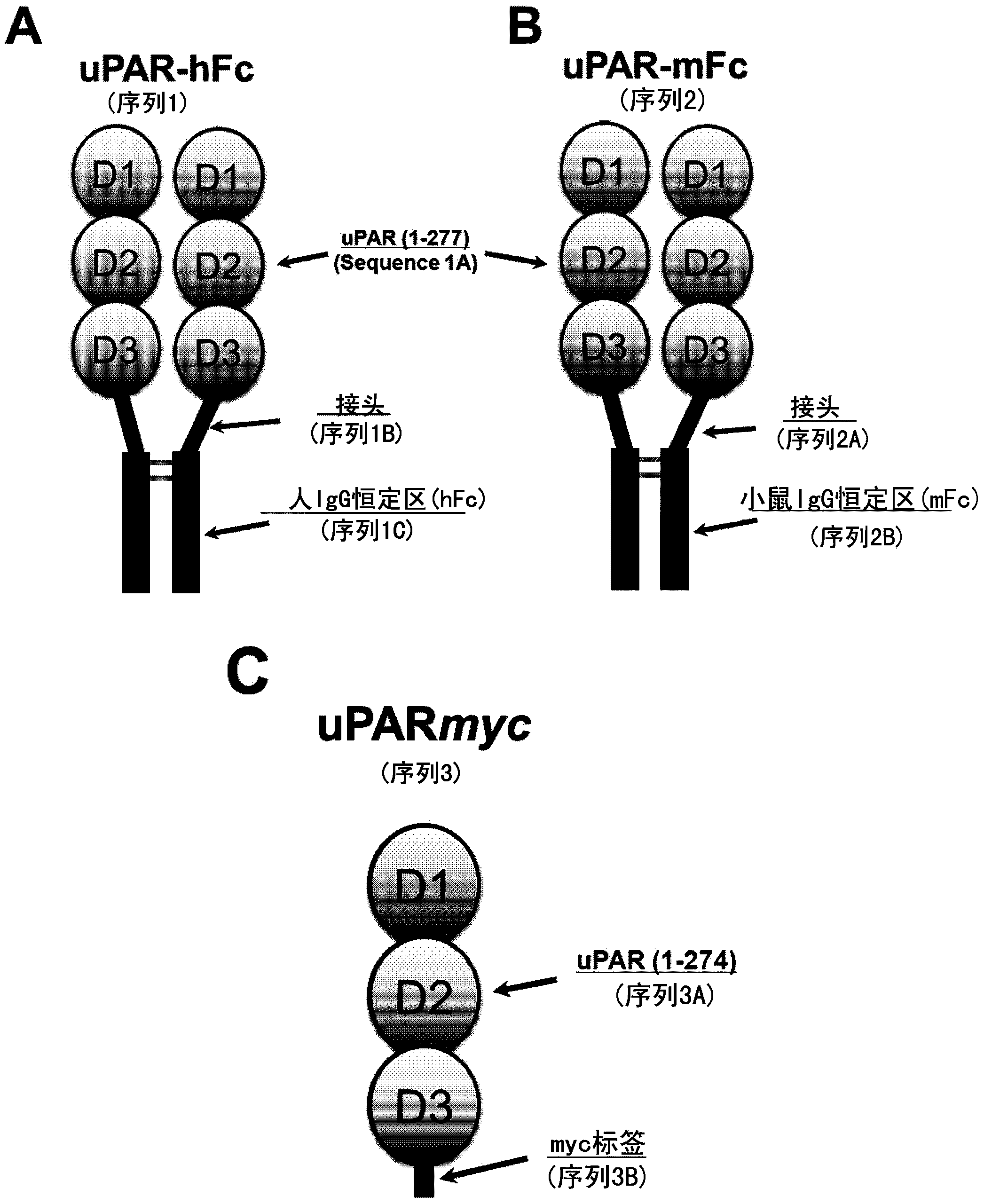
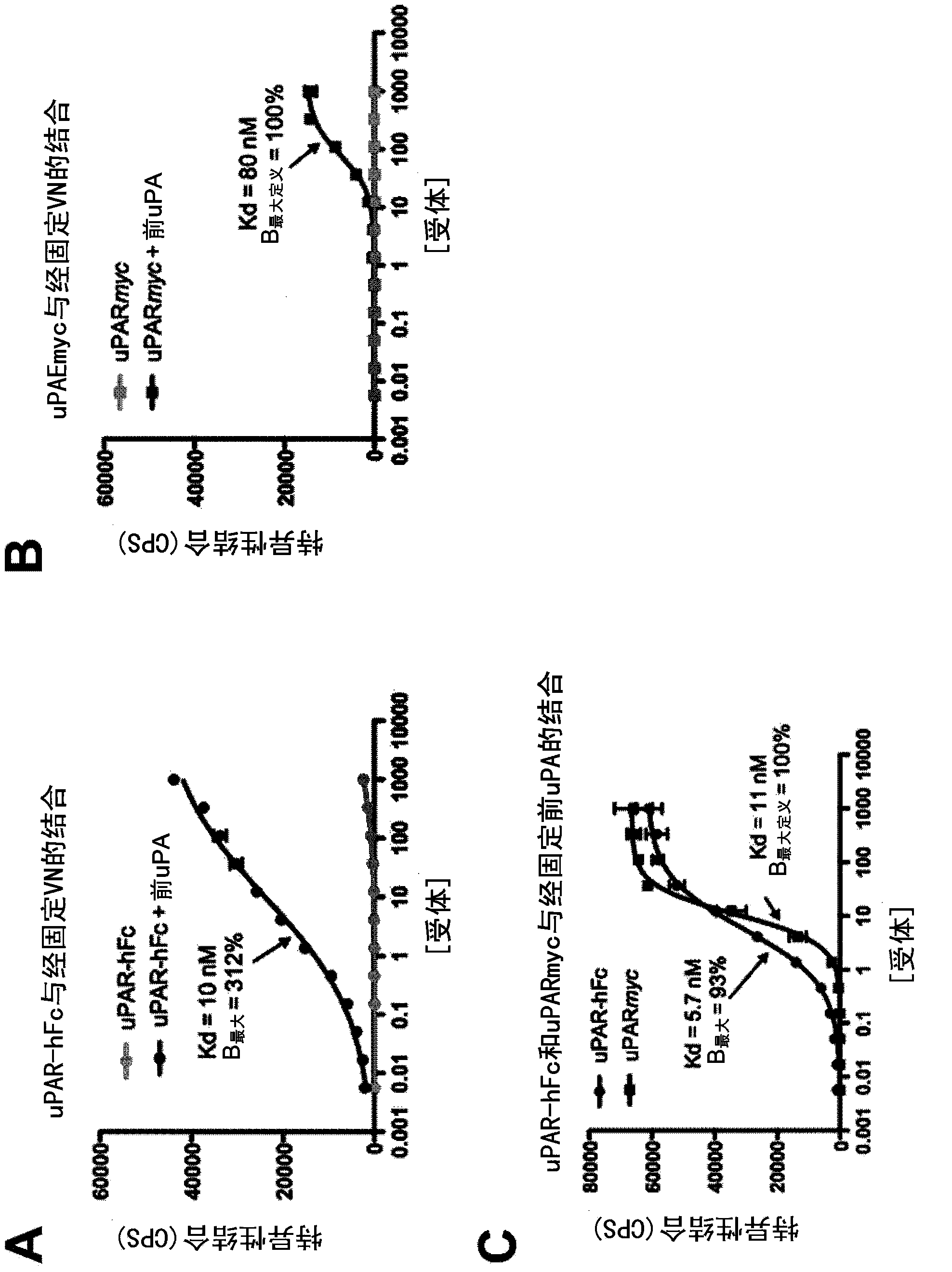
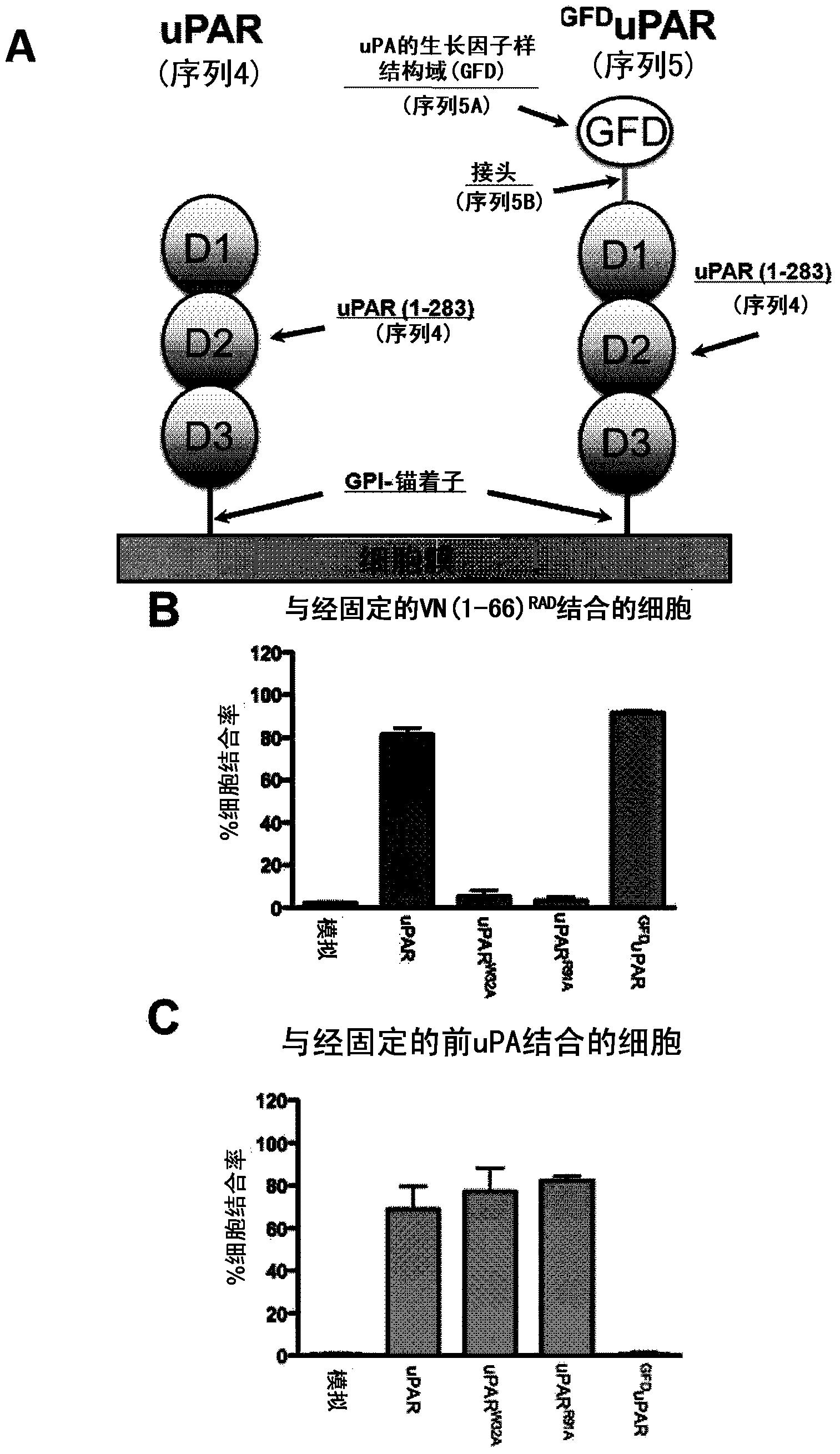
Constitutively active uPAR variants and their use for the generation and isolation of inhibitory antibodies
A variant, antibody technology, applied in the direction of antibodies, peptide/protein components, antibody mimetics/scaffolds, etc., can solve problems such as no functional blocking activity, no defined binding site, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Materials and Methods
[0112] Construction of expression vector
[0113] The expression vector for recombinant proteins with a human IgG constant region (hFc) tag was based on the pFRT / TO-Fc plasmid (Madsen et al., 2007), however, many modifications were introduced to facilitate shuffling of different coding regions and facilitate protein production. First, the XhoI restriction site located downstream of the vector sequence in the hFc coding region was destroyed by site-directed mutagenesis using oligonucleotides dXu / dXd. Second, a linker encoding the cleavage sequence of the PreScission protease prepared by annealing the oligonucleotides PreF / PreR was inserted into the XhoI site at the signal peptide / Fc junction. To remove the intron present in the Fc region of the construct (which was found to increase the yield of recombinant protein (our unpublished observation)), the vector was transfected into CHO cells, RNA was extracted, reverse transcribed , then amplified...
Embodiment 2
[0177] Materials and Methods
[0178] Antigen preparation
[0179] According to the detailed description of Example 1, the GFD uPAR-hFc and GFD uPAR-mFc was expressed and purified.
[0180] Immunization of mice
[0181] Contains 67 μg by intraperitoneal (i.p.) injection GFD 200 μl of a 1:1 emulsion of uPAR-hFc (100 μl of immunogen in PBS and 100 μl of complete Freund's adjuvant (CFA)) to immunize three 2-month-old male C57Bl / 6uPAR - / - Mice (Ms # 21574、Ms. # 1416 and Ms. # 1417). The immunized animals contained 67 μg by IP injection at 3-week intervals GFD 200 μl of a 1:1 emulsion of uPAR-hFc (100 μl of immunogen in PBS and 100 μl of incomplete Freund's adjuvant (IFA)) boosted 3 times. After a 7-week break, use 200 μg 4 days before fusion GFD uPAR-hFc in 200 μl PBS i.p. to Ms # 21574 applies a final pre-fusion immune boost. Contains 34 μg at 3-week intervals GFD 200 μl of 1:1 PBS / IFA emulsion of uPAR-hFc to Ms # 1416 and Ms. # 1417 applied three additional IP ...
Embodiment 3
[0227] Materials and Methods
[0228] mGFD Cloning of muPAR-Fc
[0229] coding mGFD The expression vector for muPAR-Fc was generated by amplifying mouse uPA cDNA with oligonucleotides muPAkf / mGFDr and mouse uPAR cDNA with oligonucleotides muL8f / MUPPFCR. The two PCR products were mixed, co-amplified with oligonucleotides muPAkf / MUPPFCR, and cloned with KpnI / XhoI into the vector pFRT / TO-Fc. The protein encoded by this vector ( mGFD muPAR-Fc, sequence 9 (SEQ ID NO: 17)) consists of 49 N-terminal residues of mouse uPA including a growth factor domain (GFD, sequence 9A (SEQ ID NO: 4)), a short linker (amino acid GGAGAAGG, sequence 9B (SEQ ID NO:8)), residues 1-273 of mouse uPAR (sequence 9C, corresponding to amino acids 1-273 of SEQ ID NO:2), the second short linker (amino acid VELEVLFQGPIE, sequence 9D (SEQ ID NO: 11)) and a human Fc tag (Sequence 1C (SEQ ID NO: 5)).
[0230] oligonucleotide sequence
[0231] muPAkf:5'-GGGGTACCATGAAAGTCTGGCTGGCGAG-3' (SEQ ID NO:54)
[02...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com