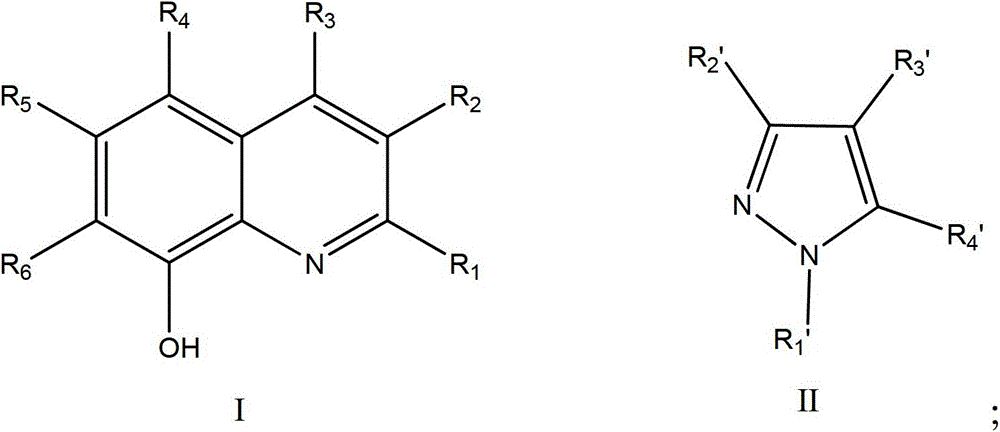
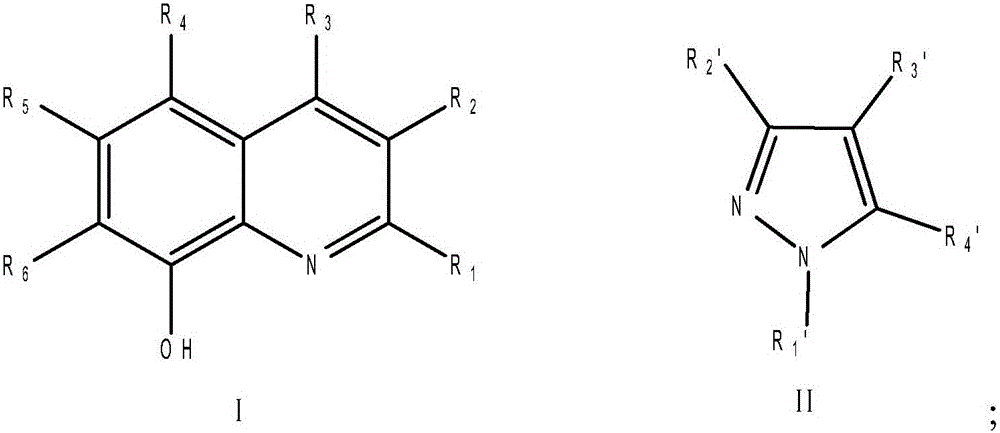
A kind of method that catalyzes the epoxidation of triazolene to prepare epoxiconazole
A technology for catalyzing triazolene and epoxiconazole, which is applied in the field of pesticides, can solve the problems of increasing post-processing steps, poor catalytic effect of olefins, and unfriendly environment, so as to simplify post-processing steps, reduce post-processing steps, and eliminate excess Effect of spent acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 0.02mmol of manganese(III) acetylacetonate, 0.06mmol of 8-hydroxyquinoline, and 0.6mmol of pyrazole into the flask, add 5mL of acetone and stir well, and then add 1mmol of triazolene. After stirring at room temperature for 10 minutes, the reaction temperature dropped to about 0°C, and 8 mmol of 30% hydrogen peroxide solution was added dropwise to the acetone solution. The addition was completed in 3 hours, and then the temperature was naturally raised to room temperature for 8 hours. The crude product of epoxiconazole was obtained with a yield of 74.2%, and a purity of 91.3% detected by Agilent 1200-HPLC (detection wavelength was 205nm, mobile phase was acetonitrile and water 50:50, C18 chromatographic column).
Embodiment 2
[0022] Add 0.02mmol of manganese(III) acetylacetonate, 0.06mmol of 5-chloro-8-hydroxyquinoline, and 0.6mmol of pyrazole into the flask, add 5mL of acetone and stir well, and then add 1mmol of triazolene. After stirring at room temperature for 10 minutes, the reaction temperature dropped to about 0°C, and 8 mmol of 30% hydrogen peroxide solution was added dropwise to the acetone solution. The addition was completed in 3 hours, and then the temperature was naturally raised to room temperature for 8 hours. The crude epoxiconazole was treated with a yield of 69.2%, and a purity of 90.8% detected by Agilent 1200-HPLC (detection wavelength was 205nm, mobile phase was acetonitrile and water 50:50, C18 chromatographic column).
Embodiment 3
[0024] Add 0.02mmol of manganese(III) acetylacetonate, 0.06mmol of 8-hydroxyquinoline, and 0.6mmol of 3-methylpyrazole into the flask, add 5ml of acetone and stir evenly, and then add 1mmol of triazolene. After stirring at room temperature for 10 minutes, the reaction temperature dropped to about 0°C, and 8 mmol of 30% hydrogen peroxide solution was added dropwise to the acetone solution. The addition was completed in 3 hours, and then the temperature was naturally raised to room temperature for 8 hours. The crude product of epoxiconazole was processed with a yield of 72.8%, and a purity of 91.0% detected by Agilent 1200-HPLC (detection wavelength was 205nm, mobile phase was acetonitrile and water 50:50, C18 chromatographic column).
[0025] NMR of the epoxiconazole product obtained above 1 H-NMR (300MHz, CDCl 3 ),δ:7.86(s,1H,azolyl-H),7.78(s,1H,azolyl-H),7.01-7.60(m,8H,Ar-H),4.72-4.76(d,1H,15Hz,CH 2 ),4.24(s,1H,CH),3.95-4.00(d,1H,15Hz,CH 2 ), its chemical shift, coupling c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com