1,7-dicyano-modified perylene imide derivatives and preparation method thereof
A technology of perylene imide and dicyano, which is applied in the field of organic chemical synthesis, can solve the problems of decreased solubility and affected device performance, etc., and achieves the effects of improving solubility, simple preparation method and excellent weather resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] (1) Preparation of 1,7-dibromo-3,4,9,10-perylenetetracarboximide (II):
[0042] Use 1 mmol of perylene tetracarbonyl diimide to correspond to 10 mL of concentrated sulfuric acid (mass concentration 98%), add perylene tetracarbonyl diimide to concentrated sulfuric acid at room temperature, stir and mix for 1 hour at room temperature, iodine catalyst, then raise the temperature to 85°C for reaction for 1 hour, add bromine water dropwise, wherein the millimolar ratio of bromine water to perylenetetracarboximide is 3; stir overnight, and pour the reaction solution after cooling to room temperature Put into ice water, precipitate the solid and filter it with suction, wash the obtained solid with water until the pH value is neutral, and obtain the solid 1,7-dibromo-3,4,9,10-perylenetetracarboximide (II);
[0043] (2) Preparation of 1,7-dibromo-3,4,9,10-perylene tetra-n-butylmethyl perylene (III):
[0044]Add 1,7-dibromo-3,4,9,10-perylenetetracarboximide (II) prep...
Embodiment 2
[0052]
[0053] (1) Preparation of 1,7-dibromo-3,4,9,10-perylenetetracarboximide (II):
[0054] Use 2 mmol of perylene tetracarbonyl diimide to correspond to 20 mL of concentrated sulfuric acid (mass concentration 98%), add perylene tetracarbonyl diimide to concentrated sulfuric acid at room temperature, stir and mix at room temperature for 1.5 hours, add iodine catalyst, then raise the temperature to 85°C for reaction for 1 hour, add bromine water dropwise, wherein the bromine water to perylenetetracarboximide millimole ratio is 3.5; stir overnight, and pour the reaction solution after cooling to room temperature Put into ice water, precipitate the solid and filter it with suction, wash the obtained solid with water until the pH value is neutral, and obtain the solid 1,7-dibromo-3,4,9,10-perylenetetracarboximide (II);
[0055] (2) Preparation of 1,7-dibromo-3,4,9,10-perylene tetra-n-butylmethyl perylene (III):
[0056] Add 1,7-dibromo-3,4,9,10-perylenetetracarboximide (II...
Embodiment 3
[0064]
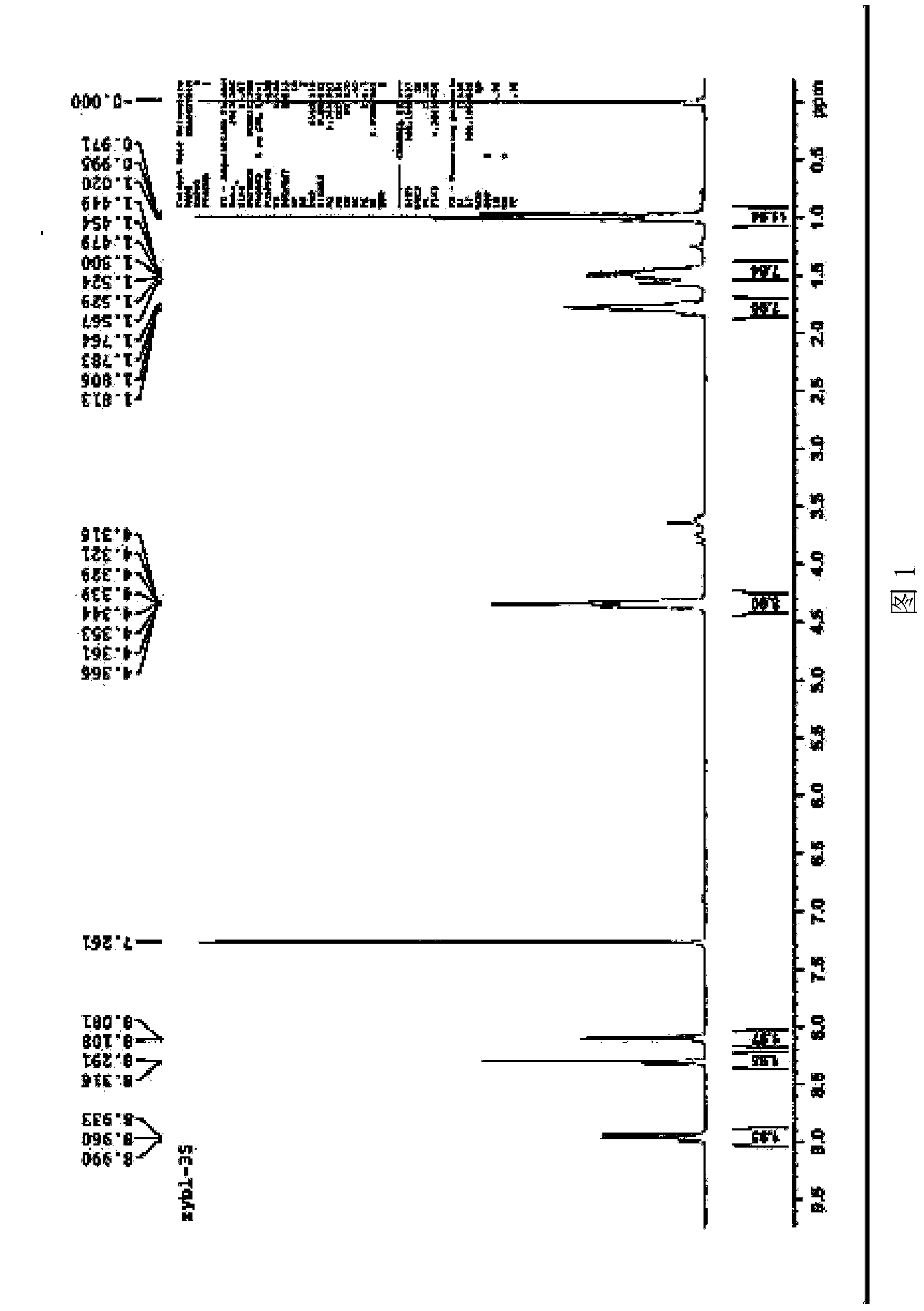
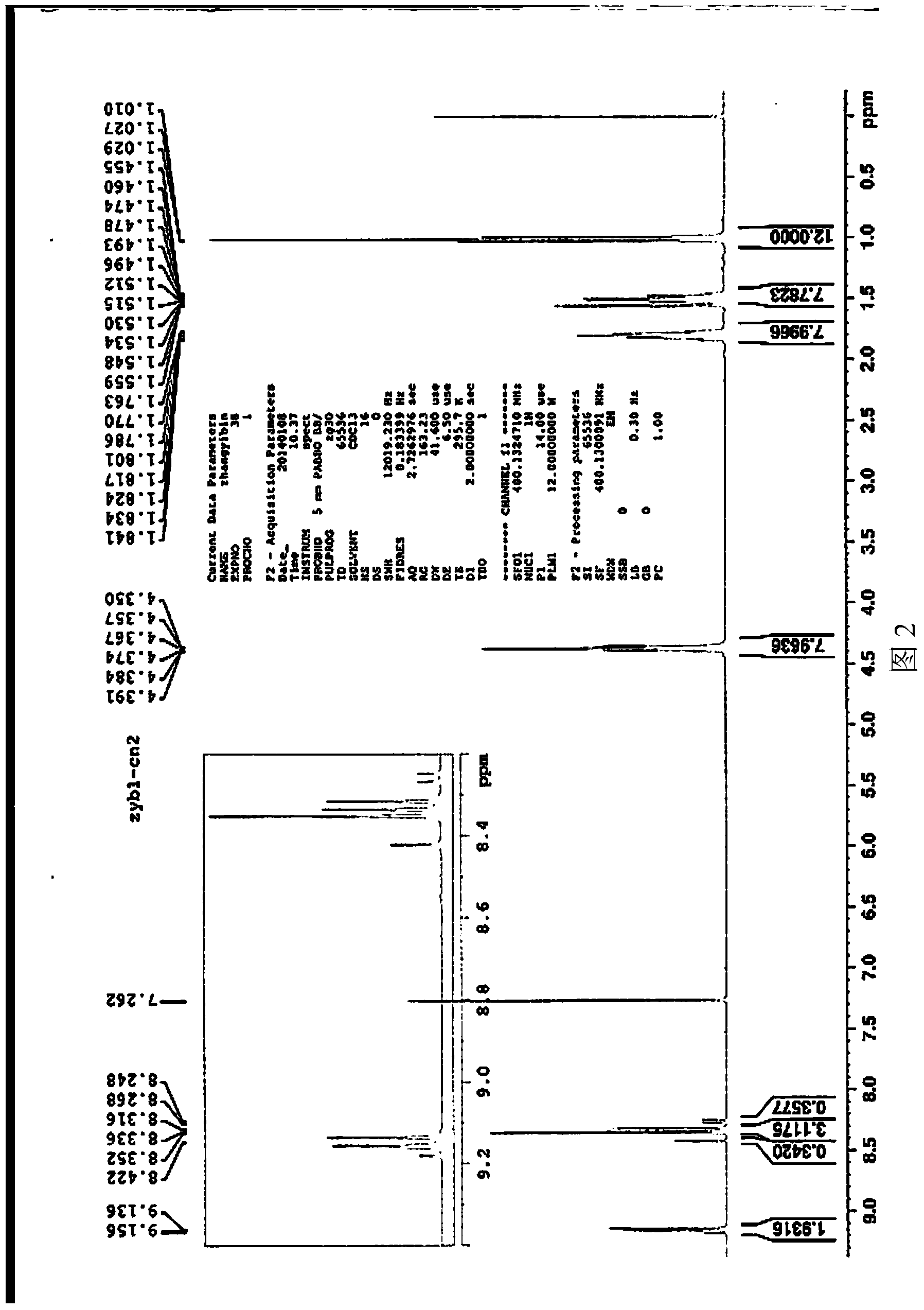
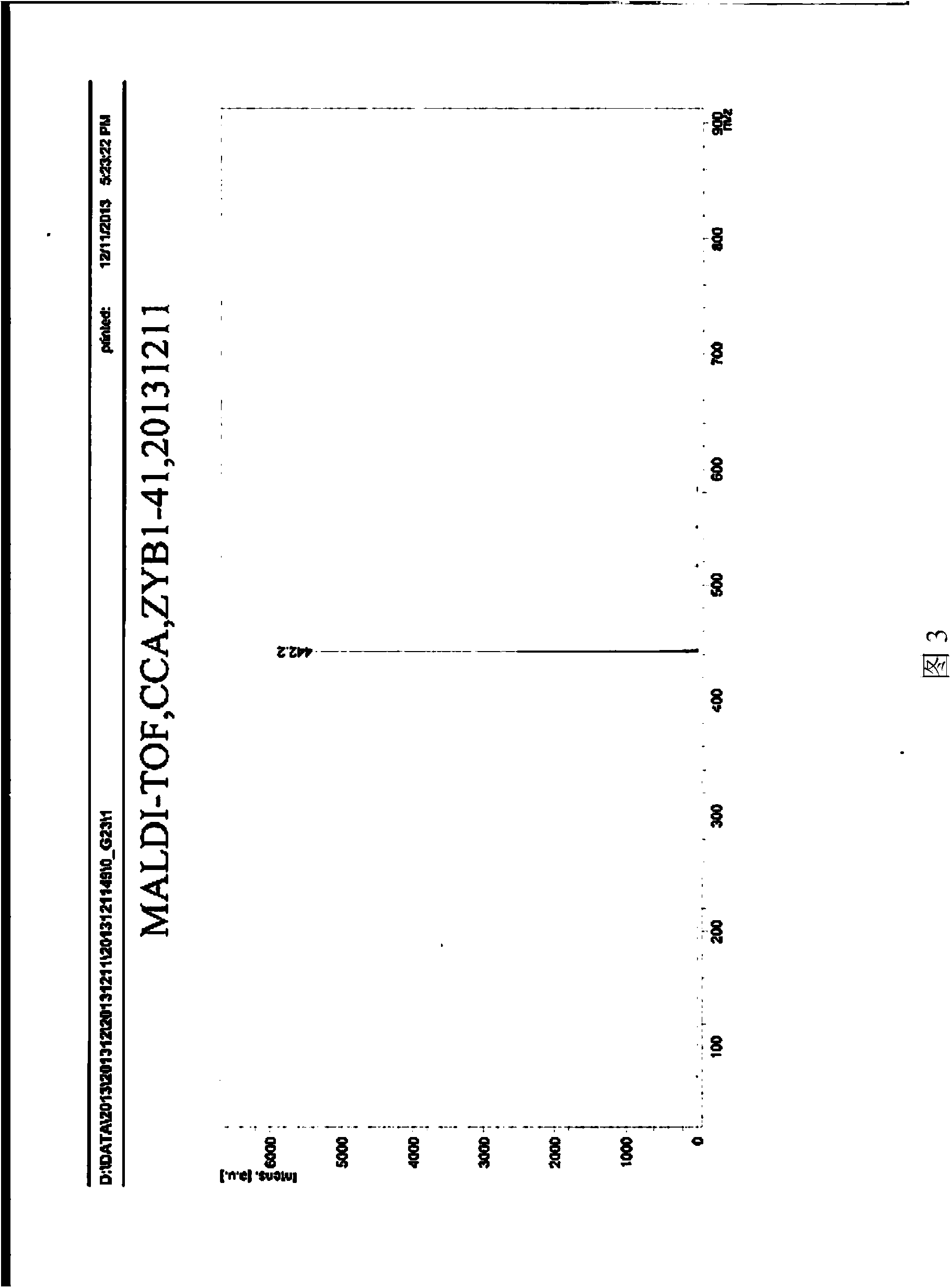
[0065] Basically the same as Example 1, except that the derivative (VI) of 1,7-dicyano-modified perylene imide is prepared as follows: the 1,7-dicyano-modified peryleneimide obtained in step (4) Imine (V) and aniline are added to the mixed solution of p-xylene and propionic acid (the volume ratio of p-xylene and propionic acid is 4:1), in which, 1,7-dicyano-modified peryleneimide The mmol ratio of aniline to aniline is 7:15; then under the protection of argon, the stirring reaction is carried out at a temperature of 140°C for 1 hour; after the reaction is completed, the obtained product is cooled to room temperature, and ethanol is added to precipitate a solid and filtered to obtain a solid After purification by DCM column chromatography, the 1,7-dicyano-modified peryleneimide derivative represented by the above formula C was obtained. NMR data spectrum see Figure 7 , mass spectrometry data spectrum see Figure 8 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com