Copolymer containing cyclopentadiene bithiophene-benzobis(benzothiadiazole), preparation method and applications thereof
A benzothiadiazole and copolymer technology, which is applied in the fields of semiconductor/solid-state device manufacturing, organic chemistry, electric solid-state devices, etc., can solve the problem of low energy conversion efficiency, wide energy band gap, and reduced photon absorption rate of organic solar cells. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
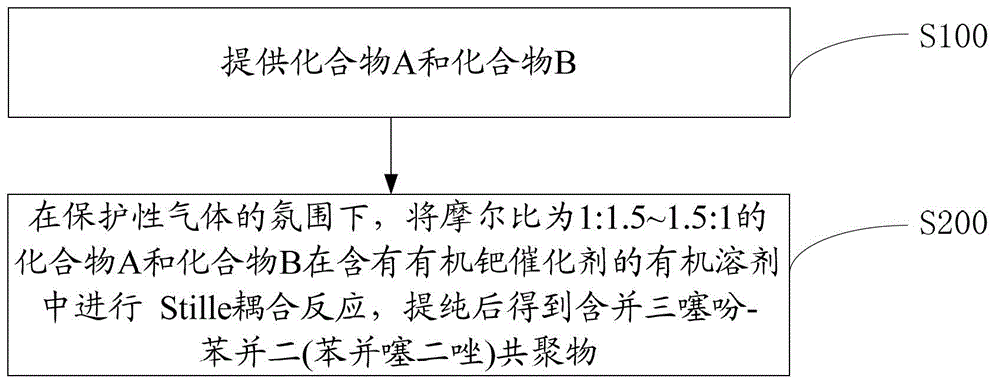
[0042] One embodiment contains the preparation method of trithiophene-benzobis(benzothiadiazole) copolymer, such as figure 1 shown, including the following steps:
[0043] Step S100, providing compound A and compound B.
[0044] A is: B is: Among them, R 1 , R 2 for C 1 ~C 20 the alkyl group, R 3 , R 4 for H or C 1 ~C 20 of alkyl.
[0045] Wherein the preparation of compound A comprises the following steps:
[0046] Step S111, adding 2-amino-5-nitroaniline to thionyl chloride (SOCl 2 ), add pyridine dropwise while stirring (the structural formula is Molecular formula is C 5 h 5 N,), wherein the solid-to-liquid ratio of 2-amino-5-nitroaniline to pyridine is 3mol:40ml, and then heated to 80°C~90°C for reflux reaction for 24 hours, then remove excess thionyl chloride, add after cooling Precipitate with deionized water, collect the solid, wash it and dry it in vacuum to obtain 5-nitro-2,1,3 benzothiadiazole.
[0047] The reaction formula of this step is:
[00...
Embodiment 1
[0092] This embodiment discloses poly{trithiophene-6,7-bis(3,7-dimethyl)octyl-benzo[2,1-e:3,4-e]bis(benzo Thiadiazole)} (containing trithiophene-benzobis(benzothiadiazole) copolymer P1):
[0093]
[0094] The preparation process of the above-mentioned trithiophene-benzobis(benzothiadiazole) copolymer P1 is as follows:
[0095] 1.1. Preparation of compound 5-nitro-2,1,3 benzothiadiazole
[0096]
[0097] Add 2-amino-5-nitroaniline (22.95g, 0.15mol) and 100ml of thionyl chloride into a three-necked flask, stir and slowly add 2ml of pyridine dropwise, heat and reflux at 80~90°C for 24h, stop the reaction , heated to 80°C and rotary evaporated excess SOCl 2 Finally, the reaction product was cooled to room temperature, poured into a large amount of water, stirred, filtered, washed with water and then vacuum-dried to obtain 21.7 g of the product 5-nitro-2,1,3-benzothiadiazole with a yield of 80%;
[0098] 1.2. Preparation of compound 4,7-dibromo-5-nitro-2,1,3 benzothiadiazo...
Embodiment 2
[0121] This example discloses poly{3,5-didecyltrithiophene-6,7-bis(3,7-dimethyl)octyl-benzo[2,1-e:3,4 -e] bis(benzothiadiazole)} (containing trithiophene-benzobis(benzothiadiazole) copolymer P2):
[0122]
[0123] The preparation process of the above-mentioned trithiophene-benzobis(benzothiadiazole) copolymer P2 is as follows:
[0124] 2.1, 4,9-dibromo-6,7-bis(3,7-dimethyl)octyl-benzo[2,1-e:3,4-e]bis(benzothiadiazole) Prepare with embodiment 1.
[0125] 2.2. Preparation of 3,5-didecyl-2,6-bis(tributyltinyl)trithiophene
[0126]
[0127] Under the protection of nitrogen, add 3,5-didecyl-2,6-dibromotrithiophene (6.35g, 0.01mol) into the three-necked flask, add 80ml of tetrahydrofuran solvent, and use the Slowly inject n-butyllithium (8.4mL, 2.5M) into the syringe, continue to stir the reaction for 1h, inject tributyltin chloride (5.6mL, 0.02mol) into the syringe at -78°C, and heat up to The reaction was stirred at room temperature for 6 hours. Saturated aqueous sodium c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com