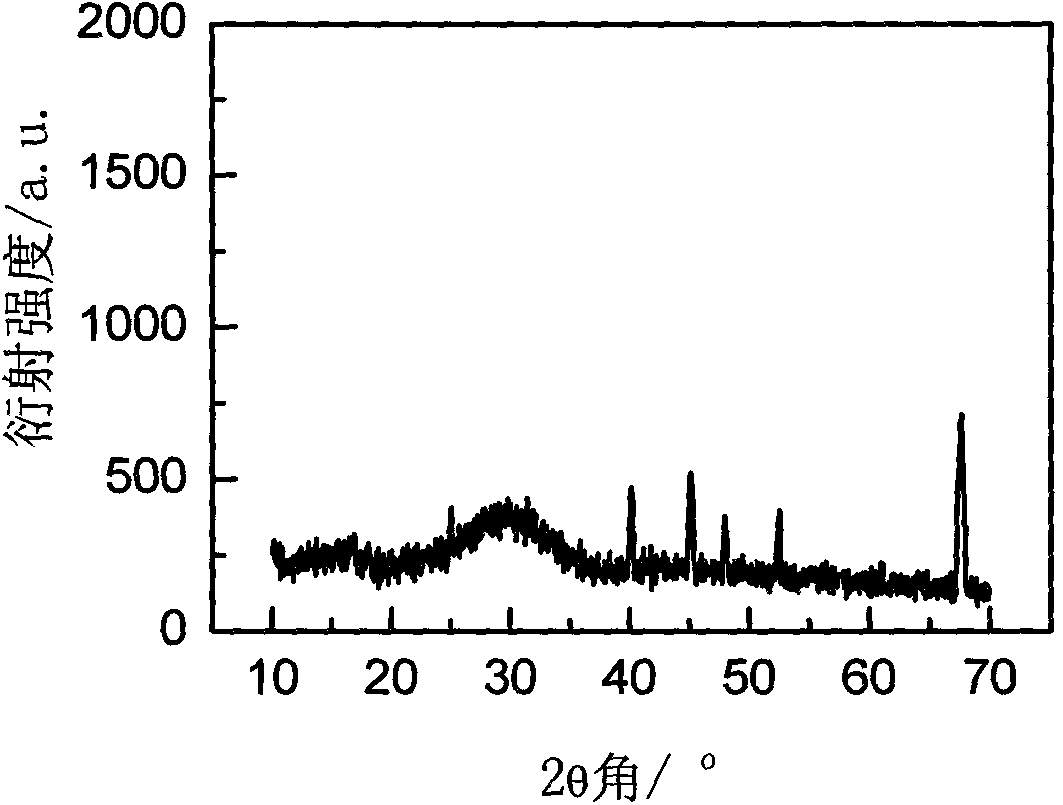
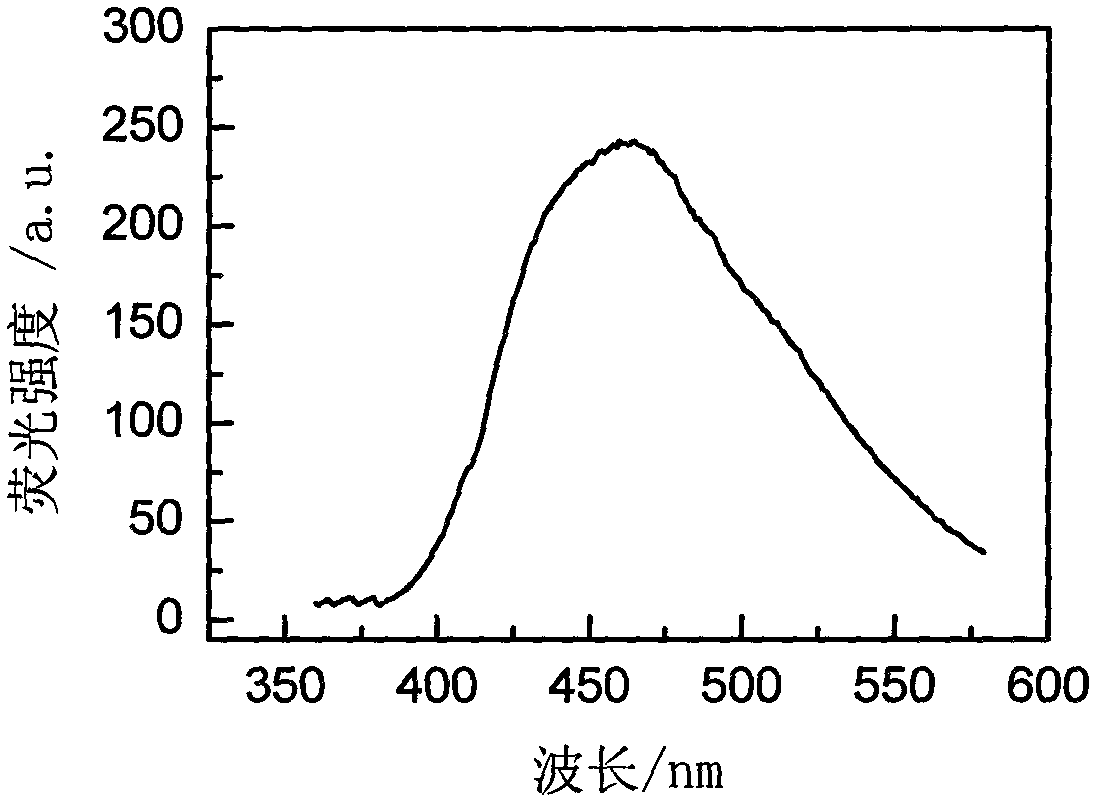
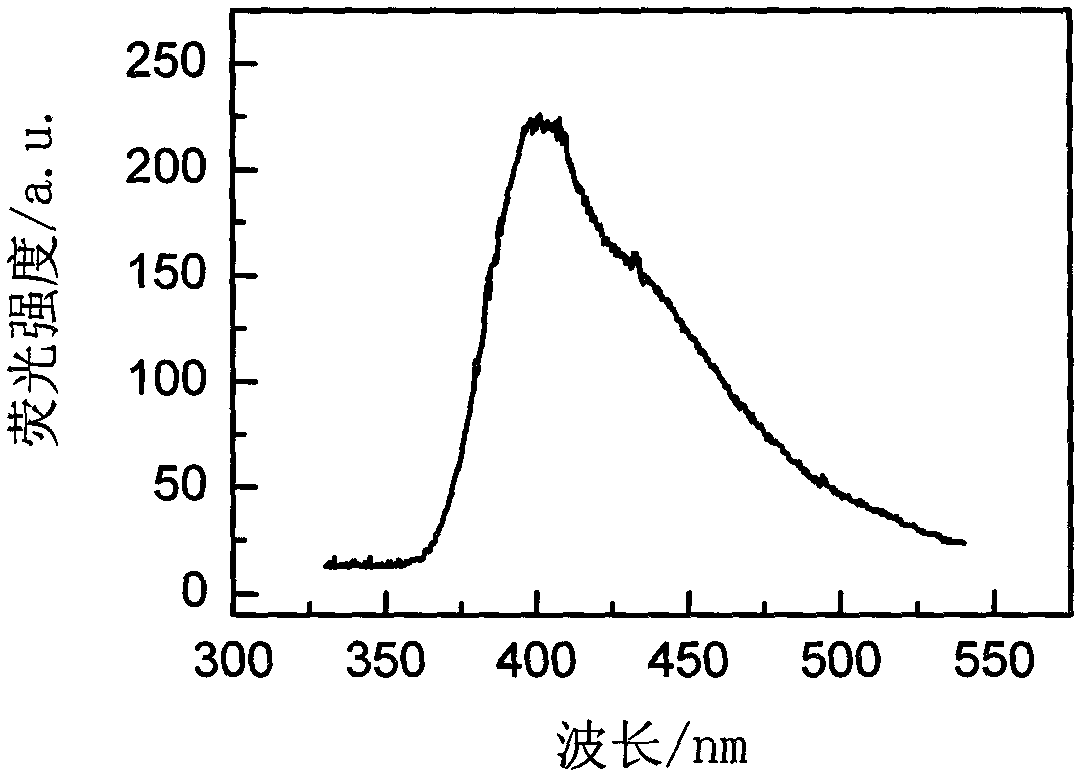
Rare-earth-ion-doped CaI2 microcrystalline glass and preparation method thereof
A technology of glass-ceramics and rare-earth ions, which is applied in rare-earth ion-doped CaI2 glass-ceramics and its preparation field, can solve problems such as difficulty in growing large-sized crystals, poor mechanical properties, and easy deliquescence of crystals, and achieve superior scintillation performance, Good mechanical properties and good permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Table 1 shows the glass formula and the first crystallization temperature value of Embodiment 1.
[0023] Table 1
[0024]
[0025] The specific preparation process is as follows: in the first step, weigh 50 grams of analytically pure raw materials according to the formula in Table 1, add 2.5 grams of NH 4 HF 2 , 2.5 g NH 4 HI 2 , Mix the raw materials evenly and pour them into a corundum crucible for melting, the melting temperature is 1450°C, keep warm for 1 hour, pour the glass melt into a cast iron mold, then place it in a muffle furnace for annealing, and keep warm at the glass transition temperature Tg for 2 Hours later, cool down to 50°C at a rate of 10°C / hour, turn off the power supply of the muffle furnace to automatically cool down to room temperature, and take out the glass; in the second step, according to the glass thermal analysis (DTA) experimental data, the first crystallization temperature of 737 ℃, heat-treat the prepared glass in ...
Embodiment 2
[0027] Embodiment 2: Table 2 shows the glass formula and the first crystallization temperature value of Embodiment 2.
[0028] Table 2
[0029]
[0030] The specific preparation process is as follows: in the first step, weigh 50 grams of analytically pure raw materials according to the formula in Table 2, add 2.5 grams of NH 4 HF 2 , 2.5 g NH 4 HI 2 , Mix the raw materials evenly and pour them into a quartz crucible to melt, the melting temperature is 1350°C, keep warm for 2 hours, pour the glass melt into a cast iron mold, then place it in a muffle furnace for annealing, and keep warm at the glass transition temperature Tg for 2 hours Hours later, cool down to 50°C at a rate of 10°C / hour, turn off the power supply of the muffle furnace to automatically cool down to room temperature, and take out the glass; in the second step, according to the glass thermal analysis (DTA) experimental data, the first crystallization temperature of 731 ℃, heat-treat the prepared glass in...
Embodiment 3
[0032] Embodiment 3: Table 3 shows the glass formula and the first crystallization temperature value of Embodiment 3.
[0033] table 3
[0034]
[0035] The specific preparation process is as follows: in the first step, weigh 50 grams of analytically pure raw materials according to the formula in Table 3, add 2.5 grams of NH 4 HF 2 , 2.5 g NH 4 HI 2 , Mix the raw materials evenly and pour them into a quartz crucible to melt, the melting temperature is 1400°C, keep warm for 1.5 hours, pour the glass melt into a cast iron mold, then place it in a muffle furnace for annealing, and keep warm at the glass transition temperature Tg for 2 Hours later, the temperature was lowered to 50° C. at a rate of 10° C. / hour, the power of the muffle furnace was turned off and the temperature was automatically lowered to room temperature, and the glass was taken out. In the second step, according to the glass thermal analysis (DTA) experimental data, the first crystallization temperature i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com