Heparin sodium injection and preparation method thereof
A technology of heparin sodium and injection, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of enhanced toxic and side effects, affecting stability, and reducing drug efficacy, so as to improve stability, The effect of maintaining stability and simple formula
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
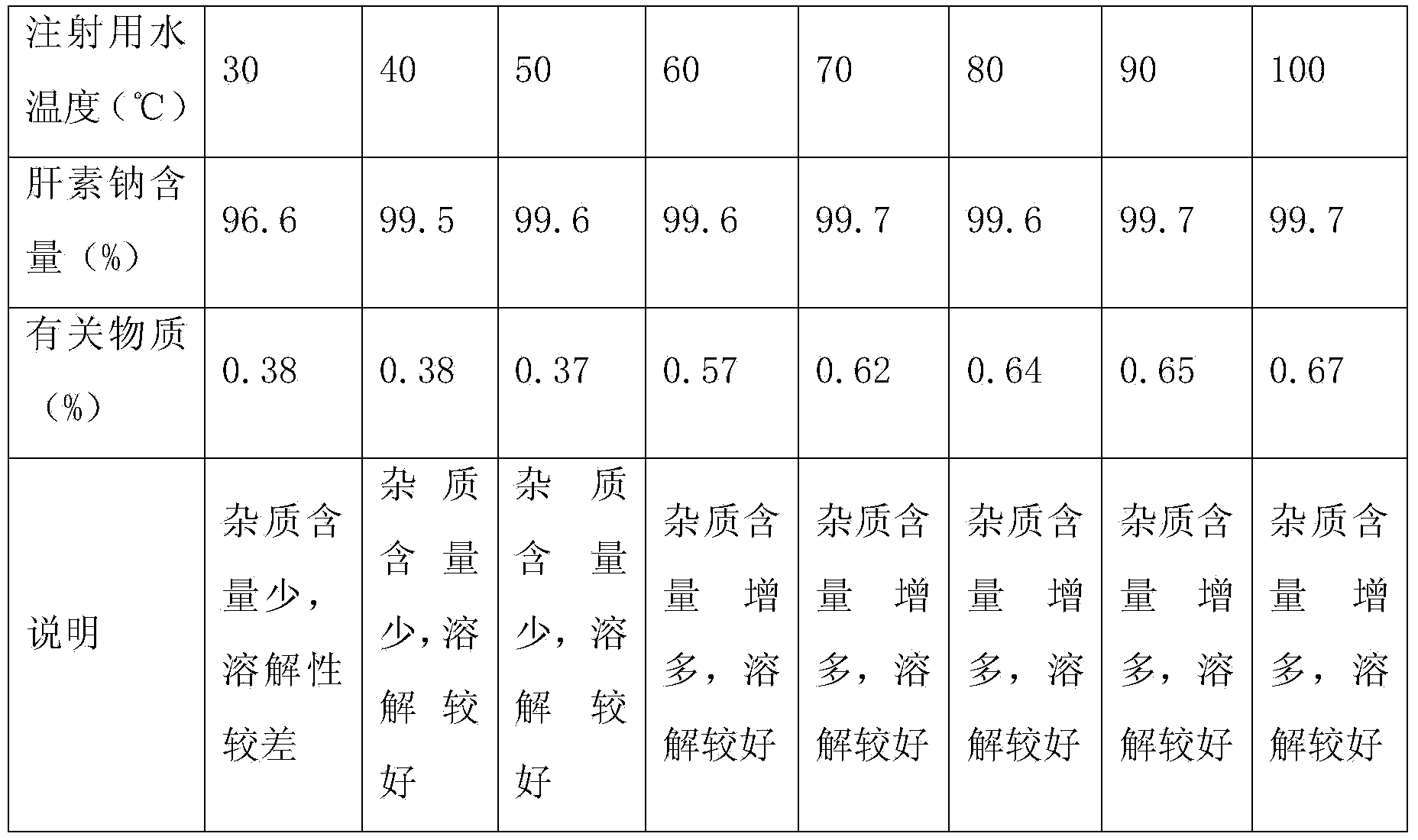
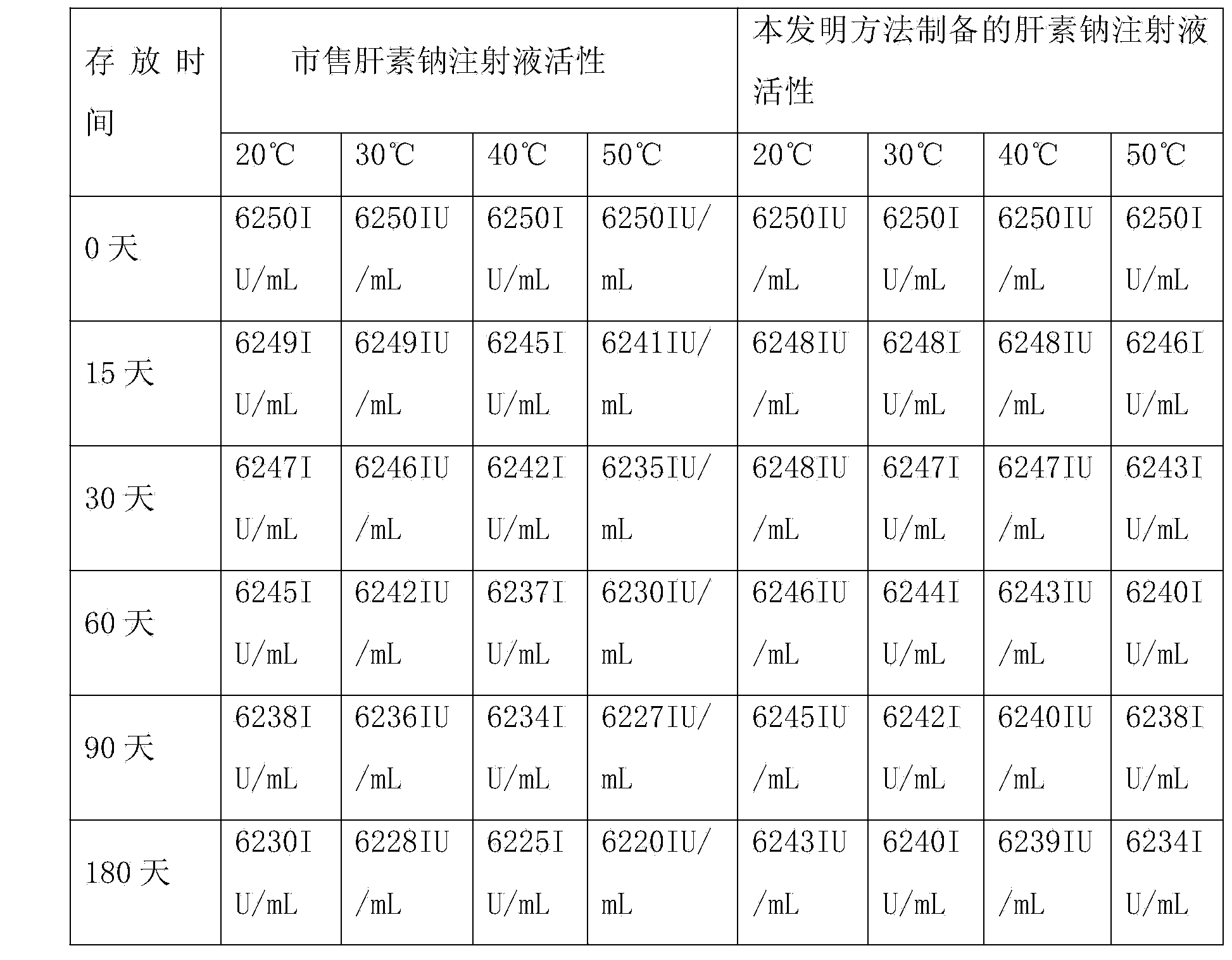
[0022] A heparin sodium injection, the injection consists of the following components:
[0023] Heparin sodium 25g, sodium chloride 80g, add water for injection to 10000ml, adjust pH to 5.8-6.8 with sodium hydroxide or hydrochloric acid.
Embodiment 2
[0025] A heparin sodium injection, the injection consists of the following components:
[0026] Heparin sodium 350g, sodium chloride 100g, add water for injection to 10000ml, adjust pH to 5.8-6.8 with sodium hydroxide or hydrochloric acid.
Embodiment 3
[0028] A heparin sodium injection, the injection consists of the following components:
[0029] Heparin sodium 50g, sodium chloride 85g, add water for injection to 10000ml, adjust pH to 5.8-6.8 with sodium hydroxide or hydrochloric acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com