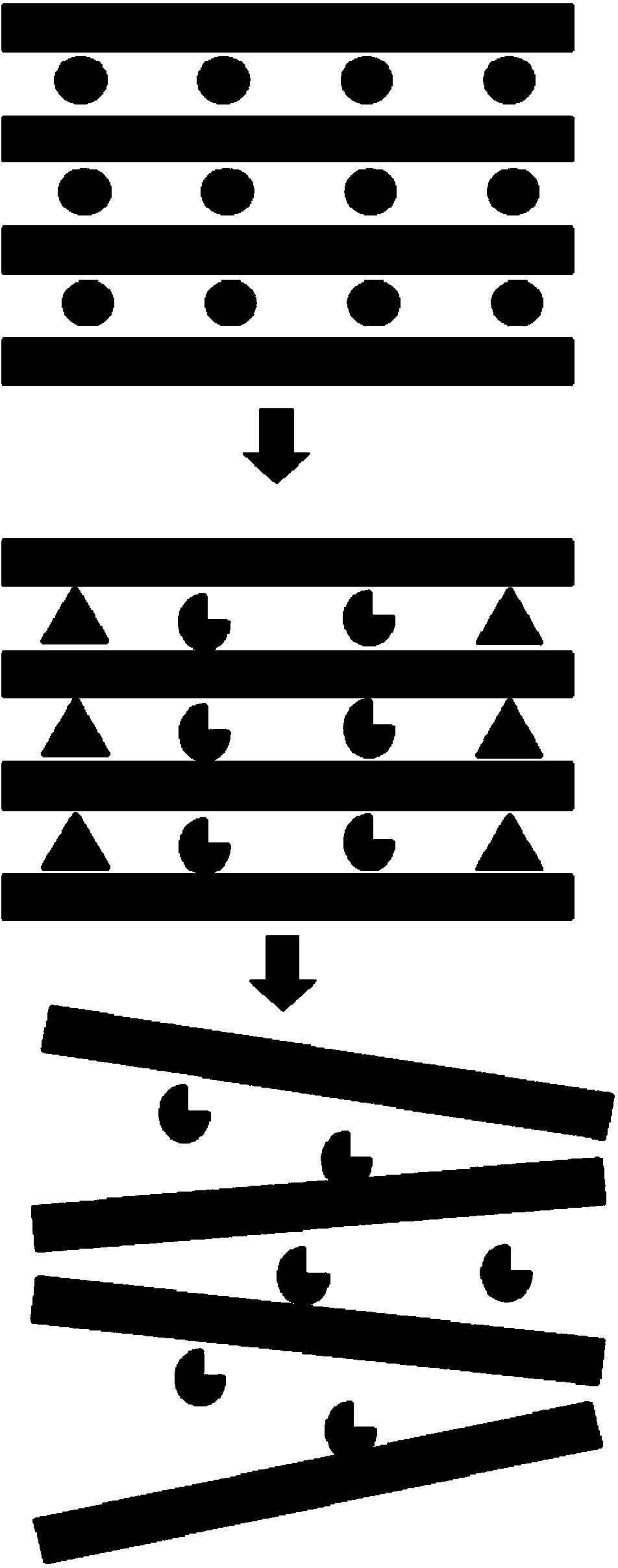
Synthesis method of bentonite loaded iron carbonyl adsorbent
A synthesis method and technology of bentonite, applied in chemical instruments and methods, adsorption water/sewage treatment, water/sludge/sewage treatment, etc., can solve the problem that the surface of hydroxyl iron cannot be fully utilized, lower utilization efficiency, slow mass transfer, etc. problems, to achieve the effect of reducing dosage, improving utilization rate and promoting adsorption speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Under the condition of stirring in a water bath at 50°C, add 0.3 mol / L NaOH solution dropwise to the ferric nitrate solution with a concentration of 1 mol / L. - / Fe 3+ =1.5, the product was aged at room temperature for 10 hours to obtain a hydroxy-iron pillar solution; the hydroxy-iron pillar solution was diluted 10 times to obtain a diluted hydroxy-iron pillar solution; dried and crushed bentonite with a 40 mesh sieve was added to the diluted hydroxy-iron pillar solution In the solution, add 3g of bentonite per milliliter of dilute hydroxyl iron pillar solution, stir for 1h, precipitate and separate, and add the solid part into the tetramethylammonium chloride solution with a concentration of 30mmol / L, and each gram of bentonite (dry weight) corresponds to 5 ml of tetramethylammonium chloride solution, stirred for 2 hours, washed with deionized water three times, and dried at 70°C to constant weight; 20 g of the dried product was placed in a microwave oven, and irradiat...
Embodiment 2
[0020] 0.5mol / L Na 2 CO 3 The solution is added dropwise to the ferric nitrate solution with a concentration of 1mol / L, and at the end of the addition, Na + / Fe 3+ =2.5, the product was aged at room temperature for 48 hours to obtain a hydroxy-iron pillar solution; the hydroxy-iron pillar solution was diluted 15 times to obtain a dilute hydroxy-iron pillar solution; the bentonite that was dried and crushed through a 100 mesh sieve was added to the dilute hydroxy-iron pillar In the solution, add 2g of bentonite per milliliter of dilute hydroxyl iron pillar solution, stir for 2h, precipitate and separate, and add the solid part into the tetramethylammonium chloride solution with a concentration of 30mmol / L, and each gram of bentonite (dry weight) corresponds to 10 ml of tetramethylammonium chloride solution, stirred for 4 hours, washed 5 times with deionized water, dried at 80°C to constant weight; 50 g of the dried product was placed in a microwave oven, and irradiated with m...
Embodiment 3
[0023] 0.4mol / L Na 2 CO 3 The solution is added dropwise to the ferric nitrate solution with a concentration of 2mol / L, and at the end of the addition, Na + / Fe 3+ =2.4, the product was aged at room temperature for 24 hours to obtain a hydroxyiron pillar solution; the hydroxyiron pillar solution was diluted 15 times to obtain a dilute hydroxyiron pillar solution; the bentonite that was dried and crushed through a 100 mesh sieve was added to the dilute hydroxyiron pillar solution In the solution, add 3g of bentonite per milliliter of dilute hydroxyl iron pillar solution, stir for 2h, precipitate and separate, and add the solid part into the tetramethylammonium chloride solution with a concentration of 100mmol / L, and each gram of bentonite (dry weight) corresponds to 10 ml of tetramethylammonium chloride solution, stirred for 4 hours, washed 5 times with deionized water, dried at 80°C to constant weight; 50 g of the dried product was placed in a microwave oven, and irradiated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com