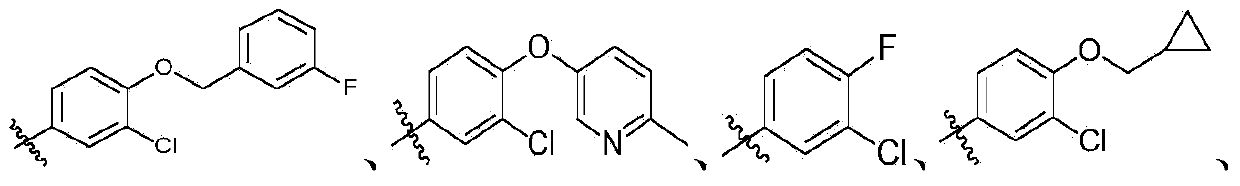
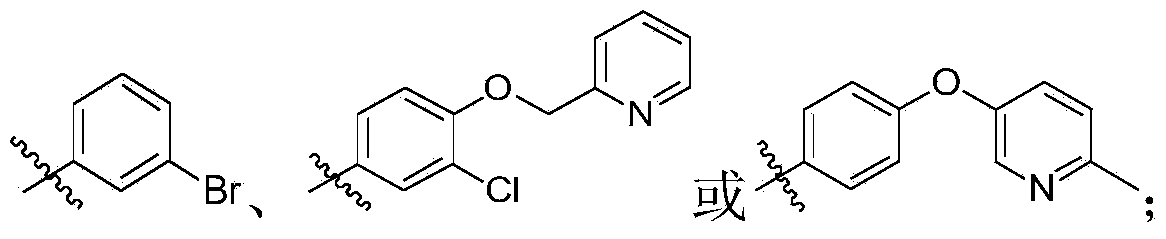
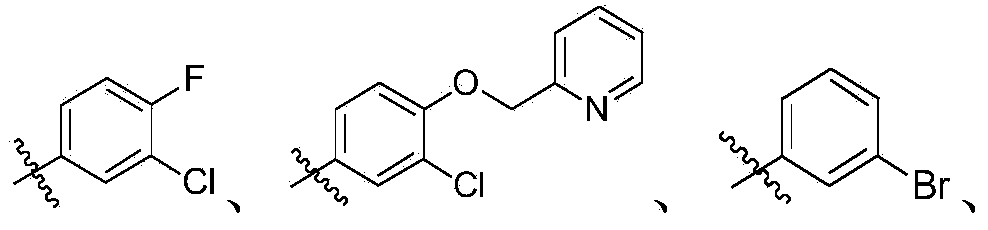
Quinoline and quinazoline derivative, preparation method, intermediate, composition and application
A technology for quinazoline and derivatives, applied in quinoline and quinazoline derivatives, preparation, intermediates, compositions and applications, capable of solving problems such as reversible inhibitor drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0367] N-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)-7-methoxyquinazolin-6-yl)-4-(dimethylamino)-2-fluorobutane Preparation of -2-enamide
[0368]
[0369] Step 1 4-(3-Chloro-4-(3-fluorobenzyloxy)phenylamino)-6-(2-fluoro-2-diethoxyphosphoacetyl)amino-7-methoxyquinazoline Preparation
[0370] Raw material: 6-amino-4-((3-chloro-4-(3-fluorobenzyloxy)phenylamino)-7-methoxyquinazoline according to the literature J.Med.Chem.2009,52,6880- Prepared by the 6888 method.
[0371] Raw material: 2-Fluoro-2-diethoxyphosphoryl acetyl chloride was prepared according to the method of Heterocycles, 2004, 63, 699-706.
[0372] Combine 6-amino-4-((3-chloro-4-(3-fluorobenzyloxy)phenylamino)-7-methoxyquinazoline (1eq.) (876mg) and triethylamine (1.5eq. ) (423μL) was dissolved in DMF (10ml), and the solution was stirred at 0°C for 30min. Add 2-fluoro-2-diethoxyphosphorylacetyl chloride (1.5eq.) (640μL) in DMF (5ml) Slowly add dropwise to the above solution, then stir overnight at room temperature. Af...
Embodiment 2
[0383] (Z)-N-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)-7-methoxyquinazolin-6-yl)-4-(dimethylamino)- 2-fluorobut-2-enamide and (E)-N-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)-7-methoxyquinazolin-6-yl )-4-(dimethylamino)-2-fluorobut-2-enamide and preparation
[0384]
[0385] The Thar SFC Pre80 supercritical preparative chromatograph was used to separate the mixture of cis and trans isomers obtained in Example 1.
[0386] Column: AD-H (20×250mm, 5μm, Tianjin Agela)
[0387] Monitoring wavelength: 254nm
[0388] Column temperature: 38 degrees
[0389] Sample dissolution: methanol dissolution, filtration
[0390] Mobile phase: ethanol (containing 0.1% DEA): carbon dioxide = 40:60
[0391] Collect the fraction with a retention time of 5.09 min to obtain the (Z)-isomer (compound 2-1); the fraction with a retention time of 8.61 min to obtain the (E)-isomer (compound 2-2).
[0392] (Z)-isomer
[0393] 1 H NMR(400MHz,DMSO)δ9.75(s,1H),9.69(s,1H),8.68(s,1H),8.53(s,1H),7.99(d,J=2.8Hz,1H),7....
Embodiment 3~11
[0397] According to the same method as in Example 1, using different raw materials, the following compounds were prepared, and the obtained products were all mixtures of cis and trans isomers.
[0398]
[0399]
[0400]
[0401]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com