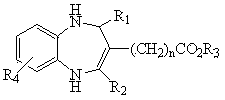
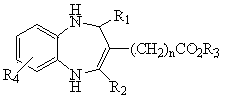
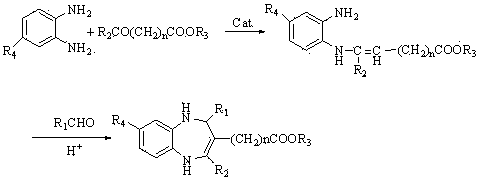
1, 5-benzodiazepine compound containing thiazolyl and ester and application of compound
A technology for benzodiazepines and compounds, which is applied in the field of medicinal chemistry, can solve the problems of no drug prospect and low bacteriostatic activity, and achieves the effects of improving pharmacokinetic properties, strong antibacterial efficacy and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 2-(2-thiazole)-3-carboethoxy-4-methyl-1,5-benzodiazepine
[0028] In a 50 mL one-necked flask, add 2 mmol of N-o-anilino-β-enaminoethyl ester and 20 mL of anhydrous methanol, then add 2 mmol of 2-thiazole aldehyde, and react at 0°C for 8 hours. Stop the reaction, let it stand, and get a solid by suction filtration, and recrystallize with absolute ethanol to get a light yellow solid, m.p: 166~168 ℃, yield 83.8%, MS [M+H + ]: 316; 1 H NMR (CDCl 3 , 500MHz) δ(ppm): 6.58-7.64 (5H, m, -C 6 h 3 , -C 3 h 2 NS), 6.11 (1H, s, -NH), 6.15 (1H, s, -CH), 4.86 (1H, s, -NH), 4.11-4.15 (2H, -COOCH 2 ), 2.60 (3H, s, -CH 3 ), 1.17-1.19 (3H, t, -CH 2 CH 3 ). IR (KBr cm-1) ν 3321 (N-H), 1680 (C=O), 1632 (C=C). The solid was determined to be 2-(2-thiazole)-3-carboethoxy-4-methyl-1,5-benzodiazepine.
Embodiment 2
[0029] Example 2 Preparation of 2-(2-thiazole)-3-carboethoxy-4-methyl-8-bromo-1,5-benzodiazepine
[0030] The same method as in Example 1 is carried out, except that N-(4-bromo-2-phenylamino)-β-enaminoethyl ester is used instead of N-o-anilino-β-enaminoethyl ester, and it needs to be reacted Concentrated under reduced pressure after completion, the residue was separated by column chromatography and rotary evaporated to obtain a light yellow granular solid, m.p: 168~170 °C, yield 87.8%, MS [M+H + ]: 394; 1 H NMR (CDCl 3 , 500MHz) δ(ppm): 6.48-7.67 (5H, m, -C 6 h 3 , -C 4 h 2 NS), 6.12 (1H, s, -CH), 6.11 (1H, s, -NH), 4.91 (1H, s, -NH), 4.11-4.15 (2H, q, -COOCH 2 ), 2.57 (3H, s, -CH 3 ), 1.17-1.20 (3H, t, -CH 2 CH 3 ). IR (KBr, cm-1) ν 3340 (N-H), 1670 (C=O), 1625 (C=C). The solid was determined to be 2-(2-thiazole)-3-carboethoxy-4-methyl-8-bromo-1,5-benzodiazepine.
Embodiment 3
[0031] Example 3 Preparation of 2-(2-thiazole)-3-carboethoxy-4,8-dimethyl-1,5-benzodiazepine
[0032] The same method as in Example 1 is carried out, except that N-(4-methyl-2-phenylamino)-β-enaminoethyl is used instead of N-o-anilino-β-enaminoethyl, and the product is light Yellow granular solid, m.p: 162~164 ℃, yield 90.1%. MS[M+H + ]: 330; 1 H NMR (CDCl 3 , 500MHz) δ(ppm): 6.40-7.65 (5H, m, -C 6 h 3 , -C 3 h 2 NS), 6.16 (1H, s, -NH), 6.12 (1H, s, -CH), 4.81 (1H, s, -NH), 4.10-4.14 (2H, q, -COOCH 2 ), 2.57 (3H, s, -CH 3 ), 2.12 (3H, s, -CH 3 ), 1.16-1.18 (3H, t, -CH 2 CH 3 ). IR (KBr, cm-1) ν 3317 (N-H), 1674 (C=O), 1621 (C=C). The solid was determined to be 2-(2-thiazole)-3-carboethoxy-4-methyl-8-methyl-1,5-benzodiazepine
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com