Application of catechol glycoside compounds to early-stage Alzhemier's disease
A technology of catechol glycosides and compounds, which is applied in the field of medicine, can solve the problems of not being able to cure Alzheimer's disease and preventing the progress of the disease, so as to improve and cognitive function, reduce oxidative stress, and improve cognition The effect of the function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthetic route of catechol glycoside compound
[0026] In the following preparations, 1 H-NMR is determined by Varian Mercury 300MHZ, and MS is determined by VG-7070. All solvents used were re-filtered, and anhydrous solvents were obtained by drying according to standard methods. The reaction was traced by TLC, and post-treatment was washed with saturated sodium bicarbonate and dried over anhydrous magnesium sulfate. Unless otherwise specified, the products were purified by column chromatography with 200-300 mesh, GF254 silica gel (Qingdao Ocean Chemical Factory) packed.
[0027] Preparation Examples:
[0028] Preparation of compound PMG:
[0029] D-anhydrous glucose 30g (167mmol), anhydrous methanol 50mL, cation exchange resin 30g, reflux for about 6h. After the reaction was complete, the resin was removed by filtration, washed with methanol several times, evaporated to dryness, and dehydrated with toluene several times to obtain 31.4 g of a whit...
Embodiment 2
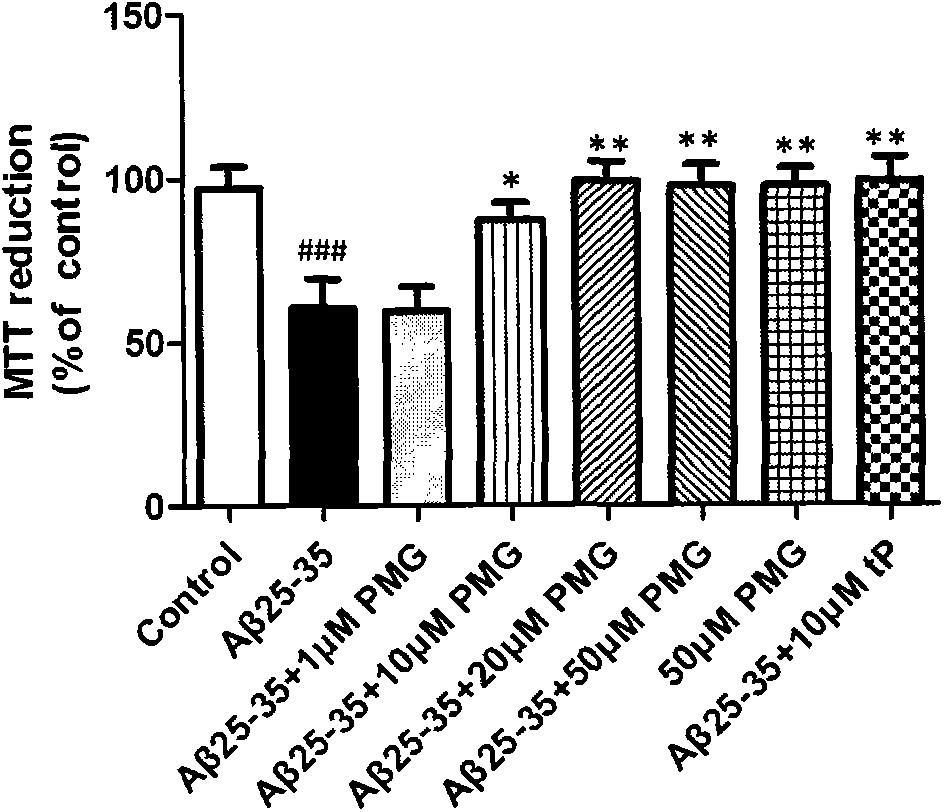
[0040] Example 2: Catechol glycosides inhibit neuronal apoptosis induced by Aβ25-35
[0041] N2a cells were cultured in DMEM medium containing 10% FBS at 37°C with 5% CO 2 cell culture incubator. Cells in the logarithmic growth phase were inoculated into 96-well plates, and PMG that had been synthesized was added. The results of MTT assay showed that N2a cells were co-incubated with 25 μM Aβ25-35 for 24 hours, and the cell viability was significantly reduced. Compared with the normal control group, the difference was the largest. obvious. There is no statistical difference in cell viability between the cell group treated with the compound alone and the cells in the normal control group, indicating that 10 μM of the compound has no obvious cytotoxicity to N2a cells. When the compound (10 μM) was co-incubated with 25 μM Aβ25-35 for 24 hours, the OD value increased, the survival rate was as high as 95.29%, and the cell survival rate was significantly higher than that of the Aβ2...
experiment example 3
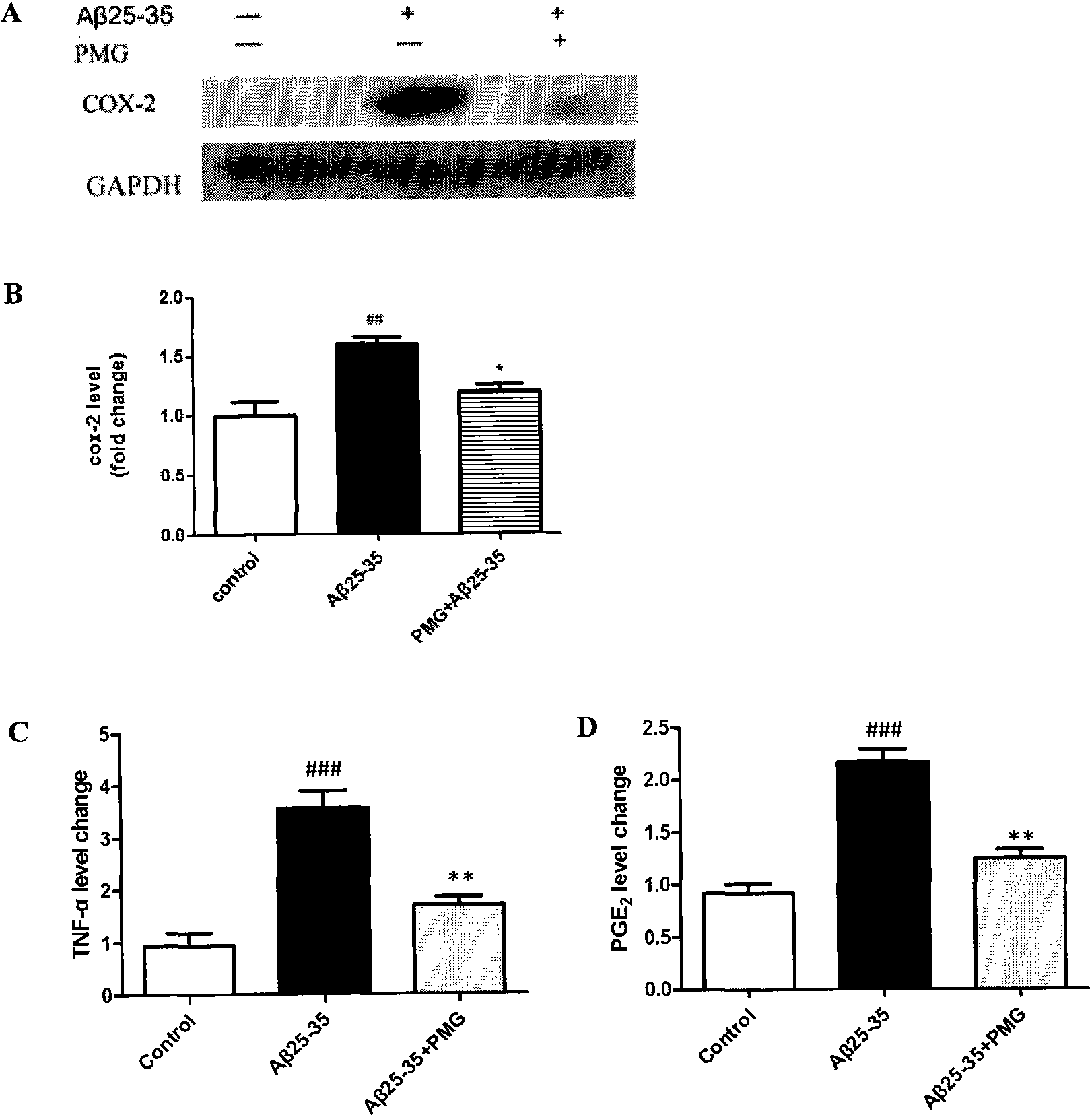
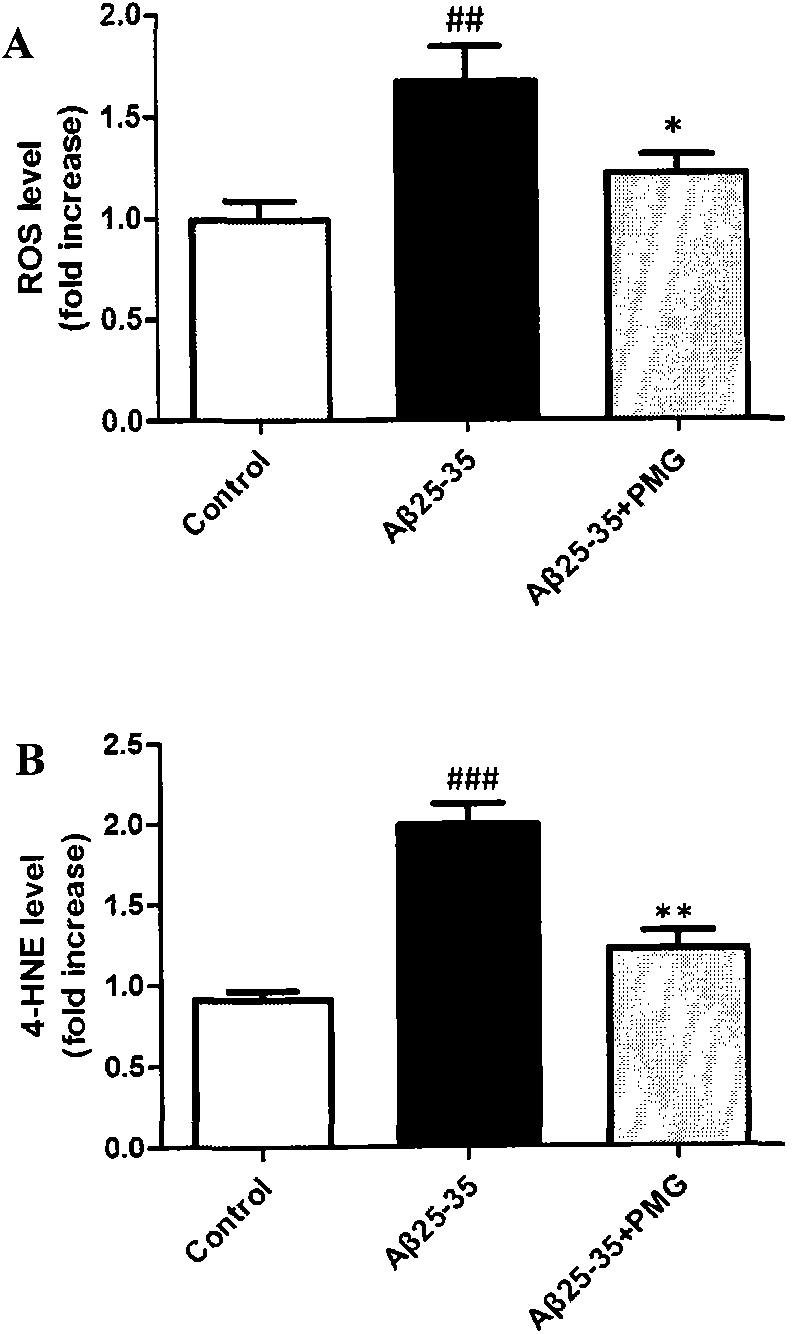
[0042] Experimental example 3: Effects of catechol glycosides on the inflammatory response of N2a cells induced by Aβ25-35
[0043] N2a cells were cultured in DMEM medium containing 10% FBS at 37°C with 5% CO 2 cell culture incubator. After the cells were treated with Aβ25-35 and PMG (10 μM) in the logarithmic phase, the cell dish was washed three times with PBS reagent, and 300 μl protein lysate (50 mM Tris-HCl (pH7.4), 150 mM NaCl, 1% NP-40, 0.1 %SDS) to lyse the cells and extract the protein. Separate the protein by SDS-PAGE electrophoresis, transfer to PVDF membrane, block with 5% milk / TBS at room temperature for one hour, add COX-2 primary antibody, spend four times overnight, wash with TBST reagent 5 times, each time for 5 minutes, then add HRP-labeled secondary antibody, which emits light after incubation at room temperature for 1 hour. For inflammatory factors TNF-α, PGE 2 For the detection of Aβ25-35 and PMG (10μM) treated cell supernatants were collected and trea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com