Lithium negative electrode with functional protective layer and lithium sulfur battery
A lithium-sulfur battery and lithium negative electrode technology, applied in the field of electrochemistry, can solve the problems of complex growth process, battery capacity attenuation, and high price, and achieve high Coulombic efficiency, small battery capacity attenuation, and strong industrial applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
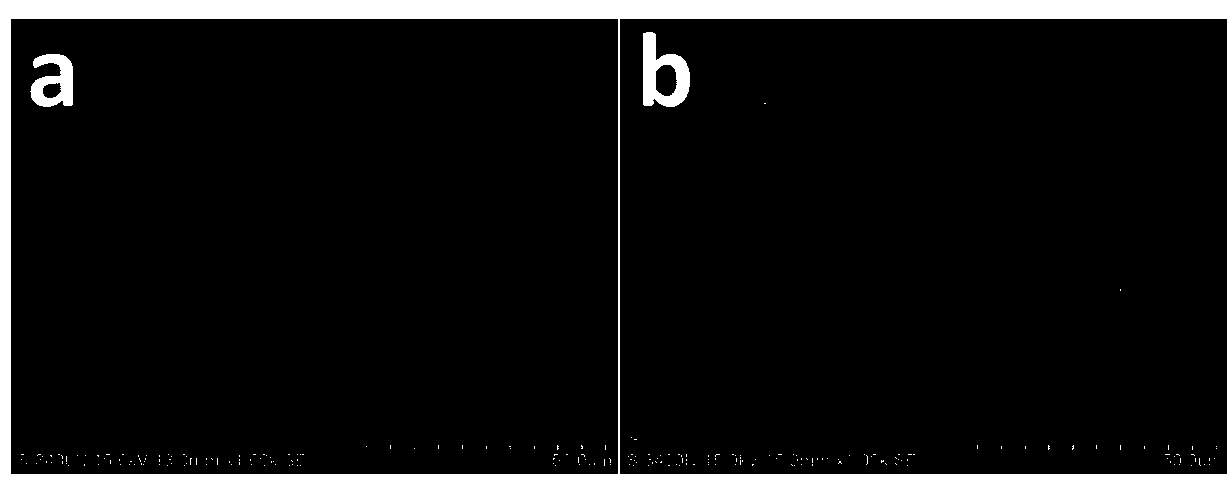
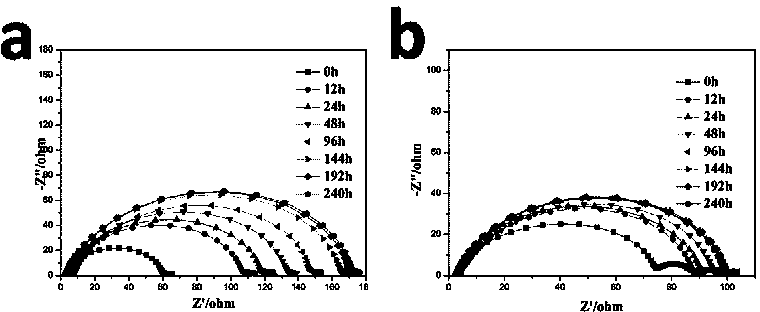
[0039] Use PEDOT-co-PEG (polyethylene glycol) copolymer nitromethane dispersion (1wt%, from sigma) to coat one side of the lithium negative electrode, place it in a glove box and dry for 30 minutes, then repeat the above process 4 times, A lithium negative electrode with a PEDOT-PEG copolymer as a protective layer was obtained, and the obtained lithium negative electrode with a protective layer / 1 M LiTFSI DOL / DME solution / the sulfur positive electrode in Comparative Example 1 was used to assemble the electrode. figure 2 Shows the surface morphology of the lithium negative electrode with PEDOT-co-PEG protective layer and the original lithium negative electrode, such as figure 2 As shown in (b), relatively speaking, the surface of the lithium negative electrode with the protective layer becomes rough. At the same time, in order to study the effect of the functional protective layer on the formation of the electrode and electrolyte SEI film, we assembled a symmetrical battery usin...
Embodiment 2
[0041] First, the polypyrrole particles are prepared by chemical methods. The specific method is: firstly prepare a 0.1M pyrrole monomer aqueous dispersion (solution A) and 0.1M ammonium persulfate solution (solution B), and then mix them under rapid stirring. Solution A was added dropwise to solution B, and the reaction continued for 12 hours at room temperature. After that, the product was washed three times alternately with deionized water and ethanol, and filtered to obtain the product. Dry at 60°C for 24h under vacuum. After that, the product was dispersed in nitromethane, and a method similar to Example 1 was used to obtain a lithium negative electrode using polypyrrole as a functional protective layer. The lithium-sulfur battery was assembled and the battery performance was tested. The results are shown in Table 1.
Embodiment 3
[0043] First, the polyaniline particles are prepared by a chemical method. The specific method is: first prepare a 0.1M aqueous dispersion of aniline monomer (solution A) and 0.1M ammonium persulfate solution (solution B), and then mix them under rapid stirring. Solution A was added dropwise to solution B, and the reaction continued for 12 hours at room temperature. After that, the product was washed three times alternately with deionized water and ethanol, and filtered to obtain the product. Dry at 60°C for 24h under vacuum. After that, the product was dispersed in nitromethane, and a method similar to Example 1 was used to obtain a lithium negative electrode using polypyrrole as a functional protective layer. The lithium-sulfur battery was assembled and the battery performance was tested. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com