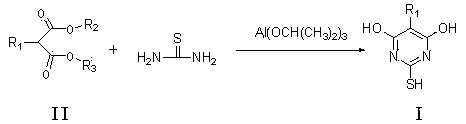Method for preparing thiobarbituric acid compound
A technology of thiobarbituric acid and compounds, applied in the direction of organic chemistry, can solve the problems of high yield and low yield, and achieve the effects of high yield, simple production, and easy factory operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
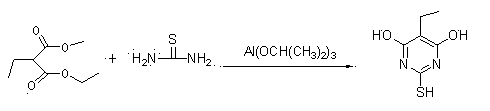
[0035] Example 1 Preparation of 4,6-dihydroxy-2-mercaptopyrimidine (using diethyl malonate as raw material)
[0036] The chemical reaction is as follows:
[0037]
[0038] Specifically: Add 152.2g (2mol) of thiourea and 2000ml of ethanol to the reactor, heat to about 50°C to dissolve; add 160.2g (1mol) of diethyl malonate, heat up to 60-70°C; within 1 hour After adding 20.4g (0.1mol) of aluminum isopropoxide, react at 70-80°C for 4 hours; slowly cool to 10-20°C and stir for 1 hour, filter; then heat to dissolve with 800ml of water, cool to 0-10°C, add hydrochloric acid to acidify to The pH was 5-7, stirred for 1 hour and filtered; vacuum-dried to obtain 130.7g. Yield 94.6%, content 98.8%.
Embodiment 2
[0044] Example 2 Preparation of 4,6-dihydroxy-5-nitro-2-mercaptopyrimidine (using 1-ethyl 3-methyl 2-nitromalonate diester as raw material)
[0045] The chemical reaction formula is as follows:
[0046]
[0047] Add thiourea 761g (10mol) and isopropanol 2000ml into the reactor, heat to about 50°C to dissolve; add 1-ethyl 3-methyl 2-nitromalonate diester 191.1g (1mol), control the temperature -10~20℃; add 204.3g (1mol) of aluminum isopropoxide within 1 hour, react at -10~10℃ for 20 hours; slowly raise the temperature to 10~20℃ and stir for 1 hour, filter; then heat and dissolve with 800ml of water, After cooling at 0-10°C, add hydrochloric acid to acidify to pH 5-7, stir for 1 hour and filter; vacuum-dry to obtain 183.9g. The yield was 97.2%, and the content detected by HPLC was 96.1%.
[0048] In addition, under the same conditions in Example 2, using sodium methoxide and sodium ethylate as the condensing agent and using aluminum isopropoxide as the condensing agent is ...
Embodiment 3
[0049] Example 3 Preparation of 4,6-dihydroxy-5-methyl-2-mercaptopyrimidine (using diethyl 2-methylmalonate as raw material)
[0050] The chemical reaction formula is as follows:
[0051]
[0052]Add 380.7g (5mol) of thiourea and 2000ml of toluene to the reactor, heat to about 50°C to dissolve; add 174.2g (1mol) of diethyl 2-methylmalonate, control the temperature at 10-20°C; Add 613g (3mol) of aluminum isopropoxide internally, react at 10-20°C for 15 hours; filter; then heat and dissolve with 800ml of water, cool down at 0-10°C, add hydrochloric acid to acidify to pH 5-7, stir for 1 hour and filter; vacuum Dry to yield 144.9 g. The yield was 91.6%, and the content detected by HPLC was 96.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com