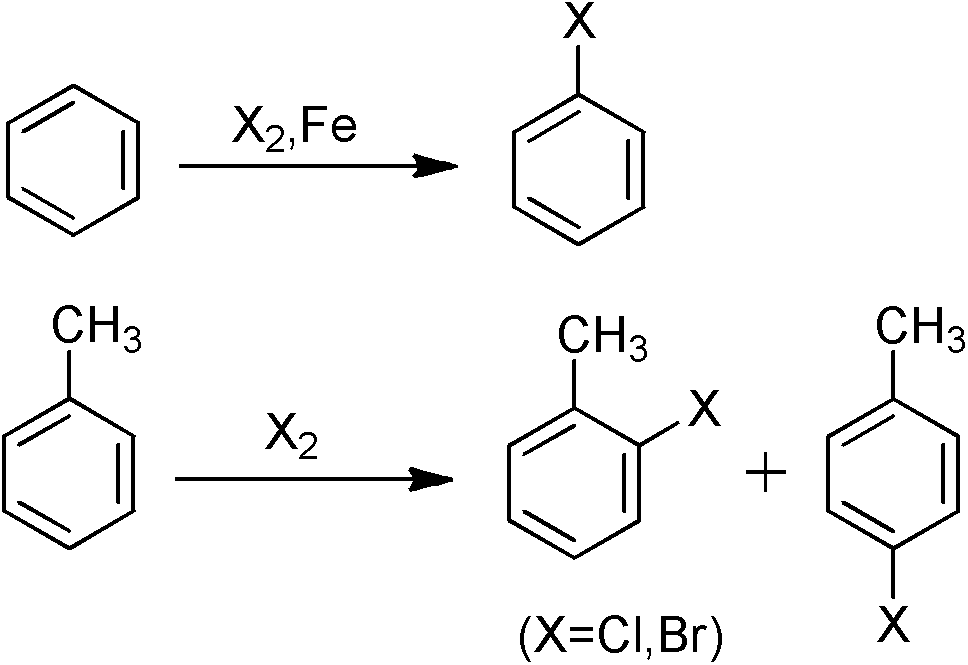
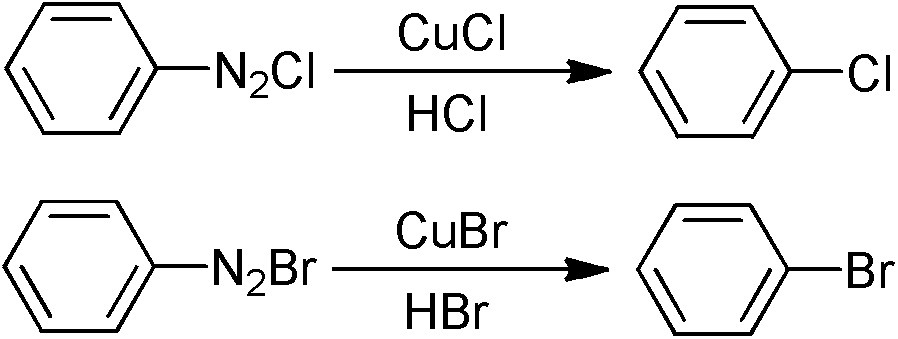
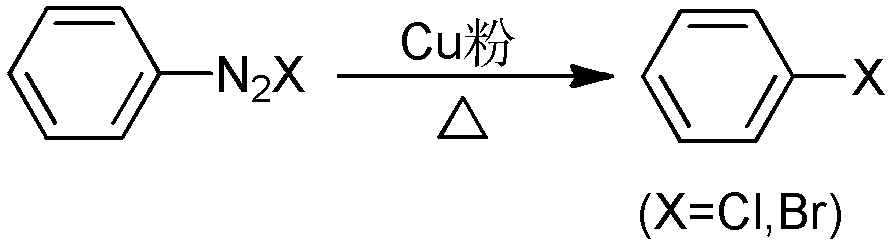
Aryl halide derivatives and synthesis method thereof
A synthesis method and derivative technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of few reports of aromatic halogen compounds, achieve high yield and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of Aryl Halide Derivatives:
[0047] The synthetic route is shown below.
[0048]
[0049] a. Precursor synthesis:
[0050] The precursor synthesis was prepared by a Cadiot-Chodkiewicz coupling reaction. Weigh 4-methyl-N, N-dipropargylbenzenesulfonamide (4.94g, 20mmol), CuCl (3.48g, 40mmol), NH 2 OH·HCl (0.28g, 4mmol) was placed in a 250mL round-bottomed flask, and in an ice-water bath, 60mL of 30% n-butylamine solution was added dropwise, stirred for 10 minutes after the addition, and then added dropwise such as 1-bromo-1 - Hexyne (9.60g60mmol), after addition, react for 4 hours; after reaction, wash with water, extract with ethyl acetate, and dry over anhydrous magnesium sulfate. The solvent was evaporated and separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate 20:1) to obtain compound 1.
[0051] b. Tandem cyclization reaction:
[0052] Weigh compound 1 (407mg, 1mmol), put it in a 10mL straight tube, add a mixed soluti...
Embodiment 2
[0062] Synthesis of Aryl Halide Derivatives:
[0063] The synthetic route is shown below.
[0064]
[0065] a. Precursor synthesis:
[0066] The precursor synthesis was prepared by a Cadiot-Chodkiewicz coupling reaction. Weigh respectively diyne compound (4.12g, 20mmol), CuCl (3.48g, 40mmol), NH 2 OH·HCl (0.28g, 4mmol) was placed in a 250mL round-bottomed flask, and in an ice-water bath, 60mL of 30% n-butylamine solution was added dropwise, stirred for 10 minutes after the addition, and then added dropwise such as 1-bromo-1 - Hexyne (12.60g, 60mmol), after addition, react for 4 hours; after reaction, wash with water, extract with ethyl acetate, and dry over anhydrous magnesium sulfate. The solvent was evaporated and separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate 20:1) to obtain compound 3.
[0067] b. Tandem cyclization reaction:
[0068] Weigh compound 3 (466mg, 1mmol), place it in a 10mL straight tube, add a mixed solution of 5mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com