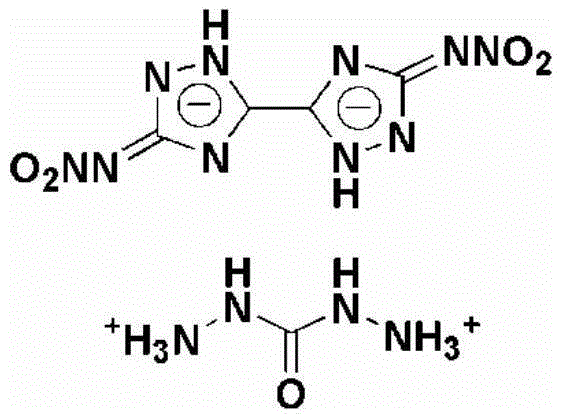
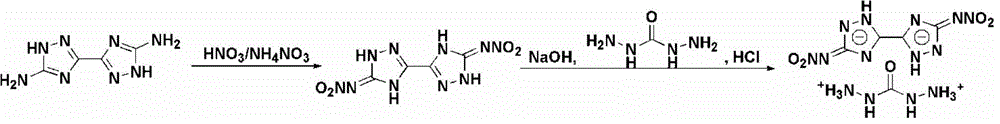
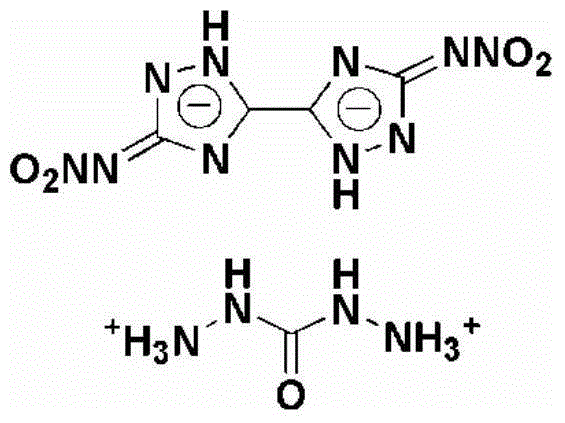
Method for synthesizing 5,5'-dinitroamino-3,3'-co-1,2,4-triazole carbohydrazide
A triazole carbohydrazide salt and a technology of triazole carbohydrazide are applied in the field of synthesis of bitriazole energetic ion salts, can solve the problems of complex steps, low total yield, difficult control and the like, and achieve a process that is easier to control, Reduce the harm to the environment and reduce the effect of exothermic reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Synthesis of 5,5'-Dinitroamino-3,3'-linked-1,2,4-triazole carbohydrazide salt
[0027] (1) Add 1g of 5,5'-diamino-3,3'-bi-1,2,4-triazole in batches to 10ml of nitric acid and ammonium nitrate system at 0°C, 5,5' -Diamino-3,3'-bi-1,2,4-triazole, nitric acid and ammonium nitrate in a molar ratio of 1:40:6.2, stirred for 15 minutes, then raised the temperature to 25°C, and reacted for 1.5 hours, The reaction solution was poured into ice water, filtered, washed and dried to obtain 1.61 g of 5,5'-dinitroamino-3,3'-bi-1,2,4-triazole.
[0028] (2) At a temperature of 60°C, 1.61g of 5,5'-dinitroamino-3,3'-bi-1,2,4-triazole, 20ml at a concentration of 1mmol·ml -1 Aqueous sodium hydroxide solution and 1.1g carbohydrazide were added to the reaction flask, 5,5'-dinitroamino-3,3'-linked-1,2,4-triazole, sodium hydroxide and carbohydrazide The molar ratio is 1:3:2.05, stir for 20min, dropwise add hydrochloric acid with a mass fraction of 35% until the pH value is 6, lower ...
Embodiment 2
[0038] (1) Add 1g of 5,5'-diamino-3,3'-bi-1,2,4-triazole in batches to 10ml of nitric acid and ammonium nitrate system at 0°C, 5,5' -Diamino-3,3'-bi-1,2,4-triazole, nitric acid and ammonium nitrate in a molar ratio of 1:40:4, stirred for 15 minutes, then raised the temperature to 25°C, and reacted for 1.5 hours, The reaction solution was poured into ice water, filtered, washed and dried to obtain 1.30 g of 5,5'-dinitroamino-3,3'-bi-1,2,4-triazole.
[0039] (2) At a temperature of 60°C, 1.30 g of 5,5'-dinitroamino-3,3'-bi-1,2,4-triazole, 15 ml at a concentration of 1 mmol·ml -1 Aqueous sodium hydroxide solution and 0.94g carbohydrazide were added to the reaction flask, 5,5'-dinitroamino-3,3'-linked-1,2,4-triazole, sodium hydroxide and carbohydrazide The molar ratio is 1:3:2.05, stir for 20min, dropwise add hydrochloric acid with a mass fraction of 35% until the pH value is 7, lower the temperature to 25°C, and the white product 5,5'-dinitroamino- 3,3'-bi-1,2,4-triazole carbohyd...
Embodiment 3
[0041] (1) Add 1g of 5,5'-diamino-3,3'-bi-1,2,4-triazole in batches to 10ml of nitric acid and ammonium nitrate system at 0°C, 5,5' -Diamino-3,3'-bi-1,2,4-triazole, nitric acid and ammonium nitrate in a molar ratio of 1:40:8, stirred for 15 minutes, then gradually increased the temperature to 25°C, and reacted for 1.5 hours , the reaction solution was poured into ice water, filtered, washed and dried to obtain 1.42g of 5,5'-dinitroamino-3,3'-bi-1,2,4-triazole.
[0042] (2) At a temperature of 60°C, 1.42g of 5,5'-dinitroamino-3,3'-bi-1,2,4-triazole, 17ml at a concentration of 1mmol·ml -1 Aqueous sodium hydroxide solution and 1g carbohydrazide were added to the reaction flask, 5,5'-dinitroamino-3,3'-linked-1,2,4-triazole, sodium hydroxide and carbohydrazide The molar ratio is 1:3:2.05, stir for 20min, add dropwise 35% hydrochloric acid until the pH value is 4, lower the temperature to 25°C, white 5,5'-dinitroamino-3, 3'-bi-1,2,4-triazole carbohydrazide salt 1.43g, the yield is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| impact sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com