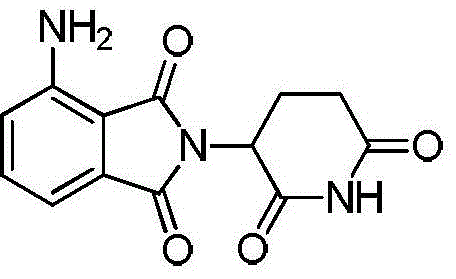
Synthetic method of pomalidomide
A technology of polilidomide and phthalimide, which is applied in the field of synthesis of new immunomodulator polilidomide, can solve the problems of high irritation, high price, and high cost, and achieve low toxicity and pollution, The effect of simple operation and stable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of polilidomide
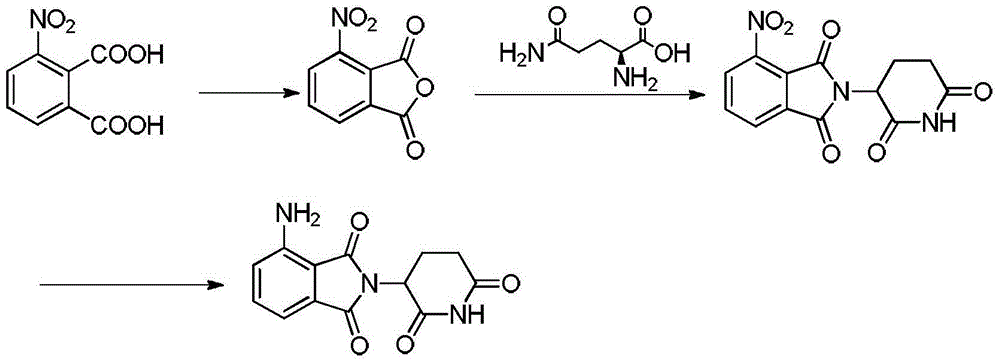
[0030] A: Preparation of 3-nitrophthalic anhydride
[0031] 3-Nitrophthalic acid (15 g, 71.05 mmol), acetic anhydride (8.7 g, 85.26 mmol), and toluene were added to the reactor. The temperature was raised to 50°C for reaction. After 20 hours of reaction, cool to room temperature, concentrate the solvent to obtain a solid and dry it, then recrystallize it with dichloromethane to obtain 13.23 g of 3-nitrophthalic anhydride after drying the obtained yellow crystal, with a yield of 98.5%.
[0032] B: Preparation of 3-nitro-N-(2,6-dioxo-3-piperidinyl)-phthalimide
[0033] 3-nitrophthalic anhydride (12g, 68.14mmol) and L-glutamine (15.14g, 103.57mmol) obtained in step A were added to the reactor, and toluene and triethylamine (5.72g, 56.49mmol) were added , heated up to 110°C and refluxed for 4.5h; after the reaction, after the solution was cooled to 60°C, acetic anhydride (6.98g, 68.35mmol) was added dropwise, the dropwise c...
Embodiment example 2
[0037] Implementation case 2: the preparation of polilidomide
[0038] A: Preparation of 3-nitrophthalic anhydride
[0039]3-Nitrophthalic acid (15 g, 71.05 mmol), acetic anhydride (54.4 g, 532.85 mmol), and toluene were added to the reactor. Raise the temperature to 110°C for reflux reaction for 1 hour; after the reaction, cool to room temperature, concentrate the solvent and dry the obtained solid, recrystallize with dichloromethane, and obtain 12.36 g of 3-nitrophthalic anhydride after drying the obtained yellow crystal, the yield 90.09%.
[0040] B: Preparation of 3-nitro-N-(2,6-dioxo-3-piperidinyl)-phthalimide
[0041] 3-nitrophthalic anhydride (12g, 62.14mmol) and L-glutamine (4.78g, 32.71mmol) obtained in step A were added to the reactor, and toluene and diethylamine (0.649g, 8.88mmol) were added , After reacting at 80°C for 5.5h, after cooling to 50°C, add acetic anhydride (47.58g, 466.05mmol) dropwise, dropwise closed and continue to heat up at 80°C for 6h; after t...
Embodiment example 3
[0045] Implementation case 3: the preparation of polilidomide
[0046] A: Preparation of 3-nitrophthalic anhydride
[0047] 3-Nitrophthalic acid (15 g, 71.05 mmol), acetic anhydride (29.01 g, 284.19 mmol) were added to the reactor. Add N,N-dimethylformamide and raise the temperature to 150°C to react for 0.5h; after the reaction, cool to room temperature, concentrate the solvent and dry the obtained solid, recrystallize with dichloromethane, and obtain 3-nitro Phthalic anhydride 8.65g, yield 63.05%.
[0048] B: Preparation of 3-nitro-N-(2,6-dioxo-3-piperidinyl)-phthalimide
[0049] 3-Nitrophthalic anhydride (12g, 62.14mmol) and L-glutamine (8.17g, 55.93mmol) obtained in step A were added to the reactor, and N,N dimethylformamide and potassium hydroxide were added (24.86mmol), heat up to 150°C for 0.5h, cool to 70°C, add dropwise acetic anhydride (31.72g, 310.7mmol), dropwise close and continue to heat up to 150°C for 1h; after the reaction, cool to room temperature, filter ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com