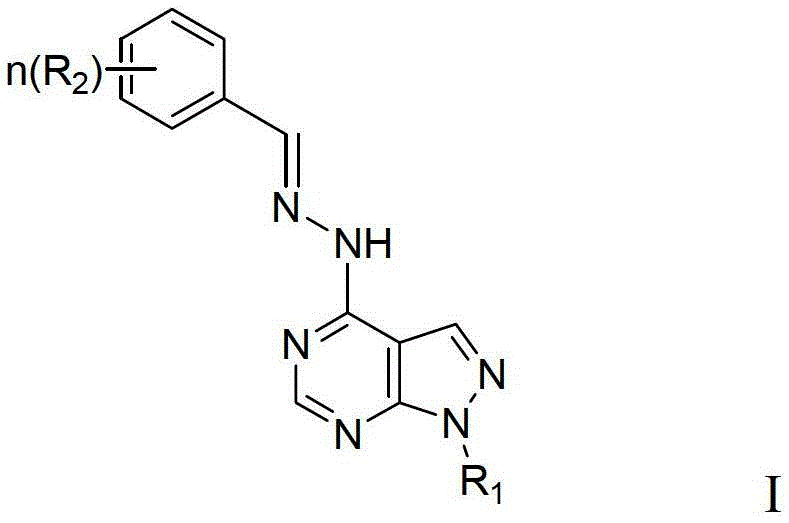
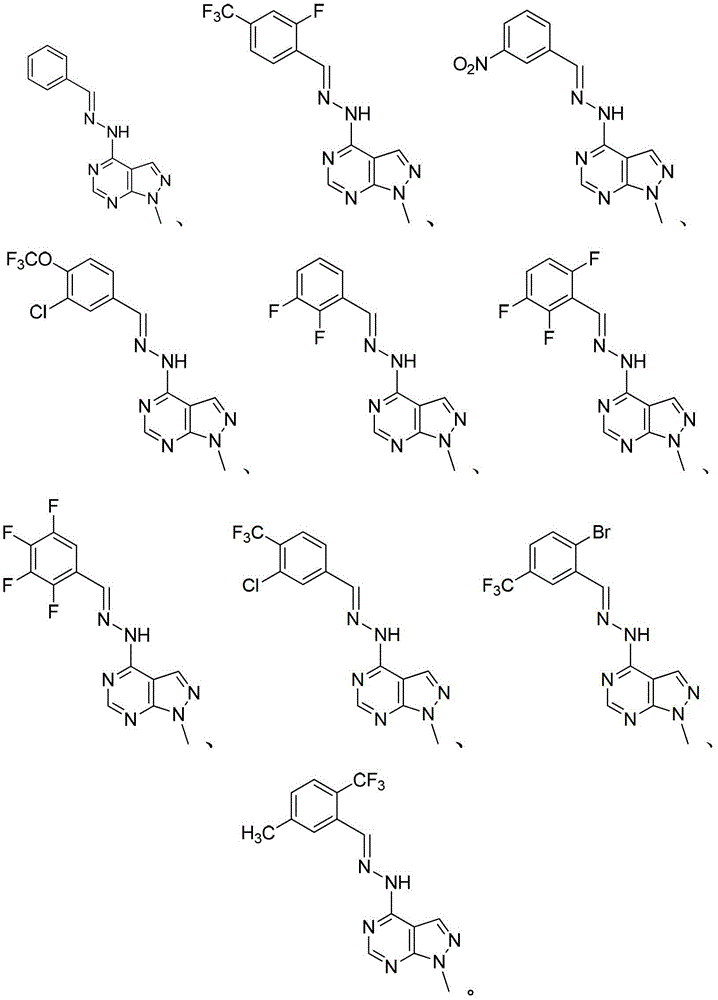
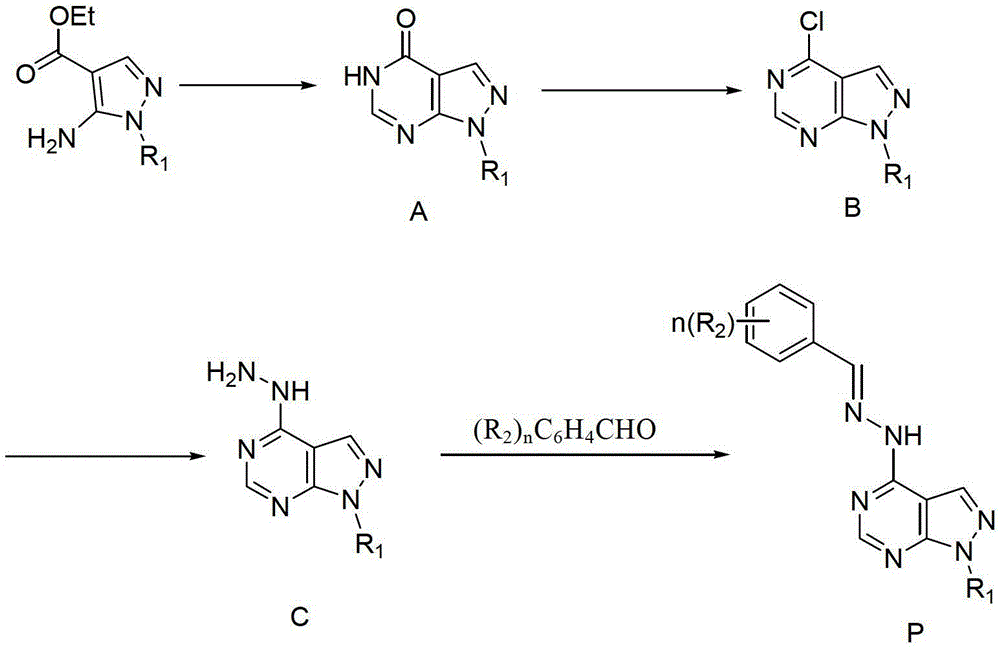
Pyrazolo pyrimidine compound and application thereof
A technology of compounds and compositions, applied in the field of agricultural science, which can solve problems such as difficulty in producing groundbreaking research results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Synthesis of Example 1.4-benzylidenehydrazino-1-methyl-pyrazol[3,4-d]pyrimidine (P-1)
[0090] (1) Synthesis of 1-methyl-pyrazol[3,4-d]pyrimidin-4-one
[0091]
[0092] Weigh 5-amino-1-methylpyrazole-4-carboxylic acid ethyl ester (2g, 11.8mmol) in a 25mL single-necked flask, add 15mL formamide, heat to 180°C and stir for 5h, then cool to Room temperature, filtered, washed with ethanol (2 x 8 mL) without purification.
[0093] (2) Synthesis of 4-chloro-1-methyl-1-hydro-pyrazol[3,4-d]pyrimidine
[0094]
[0095] Weigh 1-methyl-1-hydrogen-pyrazol[3,4-d]pyrimidin-4-one (500mg, 3.3mmol) in a 25mL three-neck flask, measure 5mL redistilled phosphorus oxychloride and add , heated to reflux for 5 h, cooled to room temperature, evaporated the solvent under reduced pressure, added the remaining solid to ice water, neutralized with saturated sodium bicarbonate, extracted with ethyl acetate (25 mL), washed the organic phase with saturated sodium bicarbonate solution and wate...
Embodiment 2
[0102] Synthesis of Example 2.4-(3-nitrobenzylidene)-hydrazine-1-methyl-pyrazol[3,4-d]pyrimidine (P-2)
[0103]
[0104] Weigh 4-hydrazino-1-methyl-1-hydro-pyrazolo[3,4-d]pyrimidine (100mg, 609μmol) in a 25mL single-necked flask, measure 10mL of absolute ethanol, add 50mg of acetic acid dropwise , heated and stirred until the raw materials were completely dissolved, weighed 3-nitrobenzaldehyde (184mg, 1.2mmol) and added to the above reaction system, continued to heat and reflux for 2.5h, then cooled to room temperature, filtered, and the filter cake was washed with absolute ethanol, free Recrystallization from water and ethanol gave 153 mg of a white solid with a yield of 84% and a melting point of 306.3-308.7°C. 1 H NMR (400MHz, DMSO-d 6 )δ12.32(s,1H),8.51(s,1H),8.40-8.36(m,3H),8.26(dd,J 1 =8.0Hz,J 2 =8.4Hz,2H),7.77(dd,J 1 =8.0Hz,J 2 =7.6Hz,1H),3.97(s,3H). HRMS(EI / [M + ]): C 13 h 11 N 7 o 2 Calculated: 297.0974; Experimented: 297.0978.
Embodiment 3
[0105] Synthesis of Example 3.4-(2-fluoro-4-trifluoromethylbenzylidene)-hydrazine-1-methyl-pyrazol[3,4-d]pyrimidine (P-3)
[0106]
[0107] Weigh 4-hydrazino-1-methyl-1-hydro-pyrazolo[3,4-d]pyrimidine (100mg, 609μmol) in a 25mL single-necked flask, measure 10mL of absolute ethanol, add 50mg of acetic acid dropwise , heated and stirred until the raw materials were completely dissolved, weighed 2-fluoro-4-trifluoromethylbenzaldehyde (234mg, 1.22mmol) into the above reaction system, continued to heat and reflux for 2.5h, then cooled to room temperature, filtered, and used for filter cake Washed with absolute ethanol, and recrystallized from absolute ethanol to obtain 181 mg of a white solid with a yield of 87% and a melting point of 247.2-249.0°C. 1 H NMR (400MHz, DMSO-d 6 )δ12.34(s,1H),8.45-8.37(m,3H),8.24(dd,J 1 =7.6Hz,J 2 =7.6Hz, 1H), 7.79(d, J=10.8Hz, 1H), 7.69(d, J=8.4Hz, 1H), 3.97(s, 3H). 13 C NMR (100MHz, DMSO-d 6 ) δ 161.4, 158.9, 156.3, 155.2, 154.0, 137.3, 134.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com