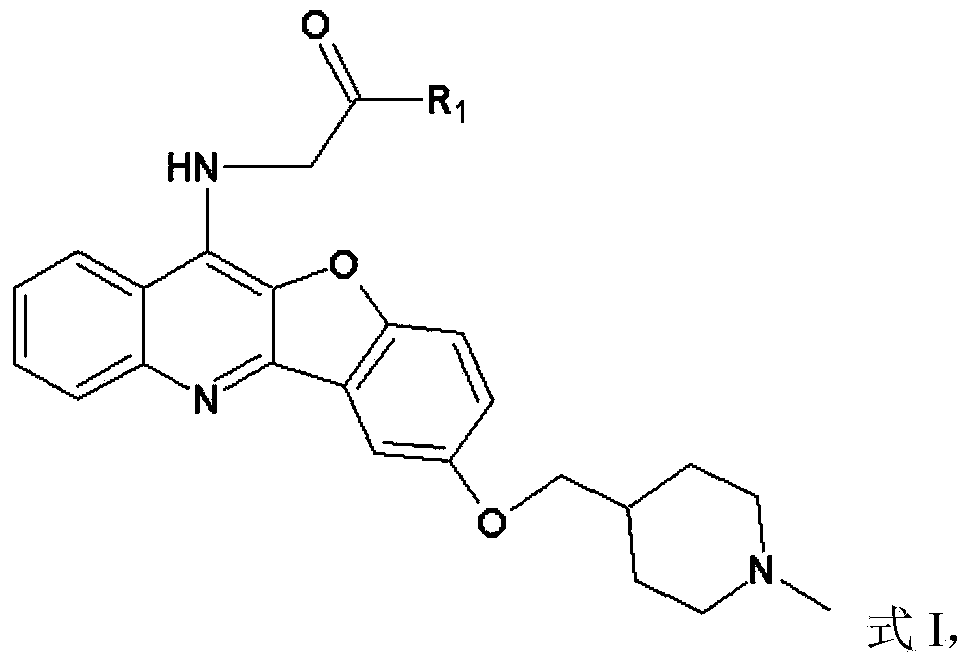
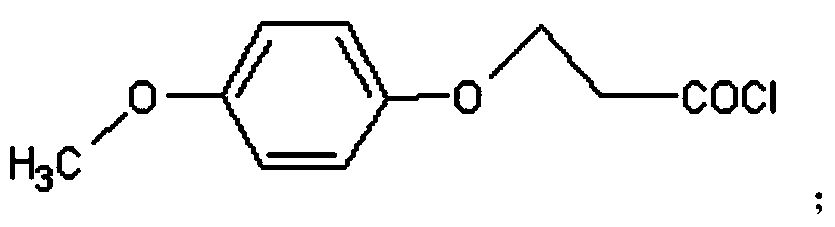
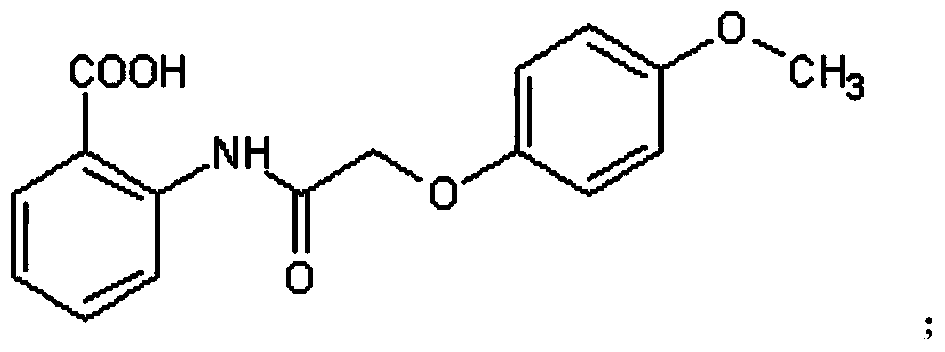
Peptidyl-substituted double-chain benzofuran quinoline derivative as well as preparation method and application thereof
A technology for benzofuranquinoline and derivatives, which is applied in the field of peptidyl-substituted double-chain benzofuranquinoline derivatives and their preparation, can solve problems such as application limitations and need to be improved, and achieves high safety and normal cytotoxicity. Small, good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment one: the synthesis of compound D
[0041]Dissolve 0.3mol of chloroacetic acid in 60ml of water, adjust the pH to 9 with sodium hydroxide, then add 0.2mol of p-hydroxyanisole, and reflux at 100°C to obtain T1, then add thionyl chloride for chlorination reaction to obtain T2 , distill off the thionyl chloride solvent to obtain a brown liquid, and then conduct condensation reaction with anthranilic acid to obtain T3, then preheat PPA to 130°C and add T3 for compound reaction to obtain compound T4, and combine T4 with thionyl chloride The chlorination reaction was carried out under reflux at 80°C to obtain compound T5, and then the 7-position methyl group was removed by using boron tribromide in dichloromethane to obtain compound T6. Then under the condition of chloroform (300mL) as solvent, add 6.0g triphenylphosphine, 2.0g T6, 6mL N-methyl-4-piperidinemethanol, 6mL diisopropyl azodicarboxylate, N 2 Under protection, diisopropyl azodicarboxylate was added dropw...
Embodiment 2
[0045] Embodiment two: the synthesis of compound DGG
[0046] Dissolve Fmoc-Gly-OH amino acid in DMF (dimethylformamide) solvent, add HOBT (1-hydroxybenzotriazole) and DIC (N,N-diisopropylcarbodiimide) for condensation Reagent (1:1), react with the deprotected Rink Amide AM resin in a solid-phase reactor for 3h, and then use 5% piperidine, 2% DBU (1,8-diazabicyclo[5.4.0]deca The mixed solution of one carbon-7-ene) and 93% DMF removes the Fmoc group to obtain the amino acid side chain, and then uses the above method to connect the Fmoc-Gly-OH amino acid to the amino acid that has been inserted into the resin.
[0047] Finally, it was condensed with D in the DMF solution of HOBT and DIC. After 72 hours, the resin was removed with trifluoroacetic acid, collected, and purified by preparative high-performance liquid chromatography to finally obtain white solid DGG.
[0048] Pale yellow solid; Yield: 43%. Melting point: 171-173°C; 1 H NMR (400MHz, DMSO-d 6 )δ9.48(s, 1H), 8.64(t,...
Embodiment 3
[0050] Embodiment three: the synthesis of compound DGR
[0051] The method is the same as in Example 2, except that the Fmoc-Arg(Pbf)-OH and Fmoc-Gly-OH amino acids are connected to the Rink Amide AM resin in sequence. Finally, after purification by preparative high performance chromatography, DGR was finally obtained as a light yellow solid.
[0052] Pale yellow solid; Yield: 31%. Melting point: 182-183°C; 1 H NMR (400MHz, DMSO-d 6 )δ9.63(s,1H),8.60(s,2H),8.16(d,J=8.0Hz,1H),8.07(d,J=5.5Hz,1H),8.00(s,1H),7.94( s,1H),7.81-7.65(m,3H),7.69(s,1H),7.44(d,J=8.8Hz,1H),7.37(s,1H),7.08(s,1H),4.73(d ,J=3.4Hz,2H),4.19(s,1H),3.99(d,J=3.9Hz,2H),3.84(s,2H),3.05(s,6H),2.79(s,3H),2.05 (d, J=14.1Hz, 3H), 1.67(s, 2H), 1.57(d, J=12.2Hz, 2H), 1.45(d, J=4.4Hz, 4H).; HRMS(ESI): m / z calcd for C 32 h 41 N 9 o 5 ([M+2H] 2+ );316.6688found316.6175.
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com