Canine single-chain antibody, and construction method and application thereof
A technology of single-chain antibody and construction method, which is applied in the biological field and can solve problems such as the difficulty of canine blood type monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
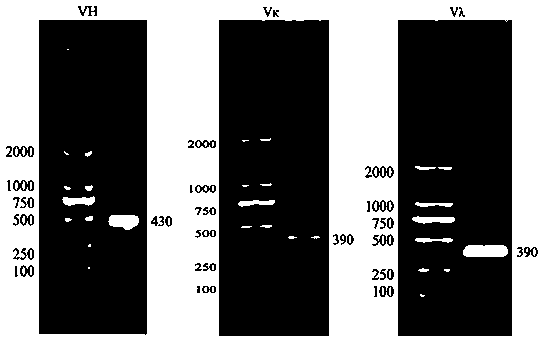
[0054] Example 1 Amplification of scFv gene
[0055] (1) Isolation of Canine Peripheral Blood Lymphocytes
[0056] Immune DEA1.1-negative dogs with canine DEA1.1-positive antigen red blood cells, and immunize 5ml of 5% red blood cells through the abdominal cavity and vein each time. or heparin) anticoagulant. Whole blood was diluted with an equal volume of PBS or 0.9% NaCl (normal saline). Add a certain volume of separation liquid into the centrifuge tube, spread the diluted blood sample above the liquid surface of the separation liquid, and keep the interface between the two liquid surfaces clear. The volume ratio of separation medium, anticoagulated undiluted whole blood, and PBS (or normal saline) is 1:1:1. At room temperature, centrifuge with a horizontal rotor at 700-800g (2000-2500rpm) for 20-30min. After centrifugation, the bottom of the centrifuge tube is red blood cells, the middle layer is the separation liquid, the top layer is the plasma / tissue homogenate lay...
Embodiment 2
[0089] Example 2 Construction and panning of phage antibody library
[0090] (1) scFv linked to pCANTAB-5E
[0091] Proceed as follows:
[0092]
[0093] Ligate at 16°C for 12-16 hours, and extinguish T4 ligase at 70°C for 10 minutes.
[0094] (2) Preparation of electrotransformation competent bacteria TG1
[0095] Resuspend the ligated vector in 1 mL LB medium. Incubate overnight at 37°C, 250rpm. Inoculate into MMP (minimal medium plate) medium and culture overnight (12h) at 37°C. Pick a single colony and culture in 5mL LB medium, 37°C, 250rpm, 10h. Pipette 900 μL of stable growth phase Ecoli . For TG1 cells, add 250 μL sterilized 80% glycerol, mix well and store at -70°C. Specifically include the following steps:
[0096] A. Pick a single colony from the micro-medium plate, inoculate it in 10mL 2×YT medium, and cultivate it overnight at 37°C with shaking;
[0097] B. Dilute the above-mentioned overnight bacteria 1:100 and inoculate them into 1000mL fres...
Embodiment 3
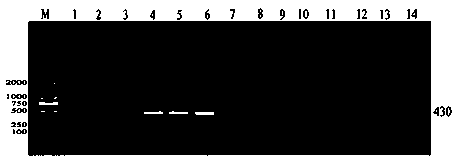
[0128] Example 3 Detection of Positive Phage Single-Chain Antibody
[0129] (1) Positive phage antibody gene amplification and sequencing
[0130] A. Add 400 μL of 2×YT-AG medium to 96 tubes, randomly pick 96 single clones from the SOBAG plate after the third round of elutriation, and culture them overnight at 200 rpm at 30°C;
[0131] B. Add 400 μL of 2×YT-AG medium to a new tube, take 40 μL of overnight cultured cells into a new tube, and save the remaining bacterial solution in glycerol for later use;
[0132] C. Take a new tube and incubate at 30°C for 2 hours at 200 rpm. Centrifuge at room temperature at 1500g for 20min, discard the supernatant;
[0133] D. Centrifuge to prepare 50mL 2×YT-AI medium, transfer 400μL to each tube;
[0134] E. Incubate at 30°C for 3-4 hours, 200rpm. Centrifuge at 1500 g for 20 min at room temperature;
[0135] F. Carefully transfer 320 μL of supernatant to a new tube. Add 80 μL blocking solution to block 320 μL supernatant, incuba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com