Method for preparing 2-methyl-1-acetenyl-2-amylene-1-ol
A technology of ethynyl and methyl, which is applied in the field of preparation of 2-methyl-1-ethynyl-2-penten-1-ol, can solve the difficulty of feeding solid ammonium chloride, high concentration of sodium hydroxide solution, Problems such as high production cost and energy consumption, to achieve the effect of reducing hydrolysis time, reducing the steps of solvent recovery, and simplifying operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
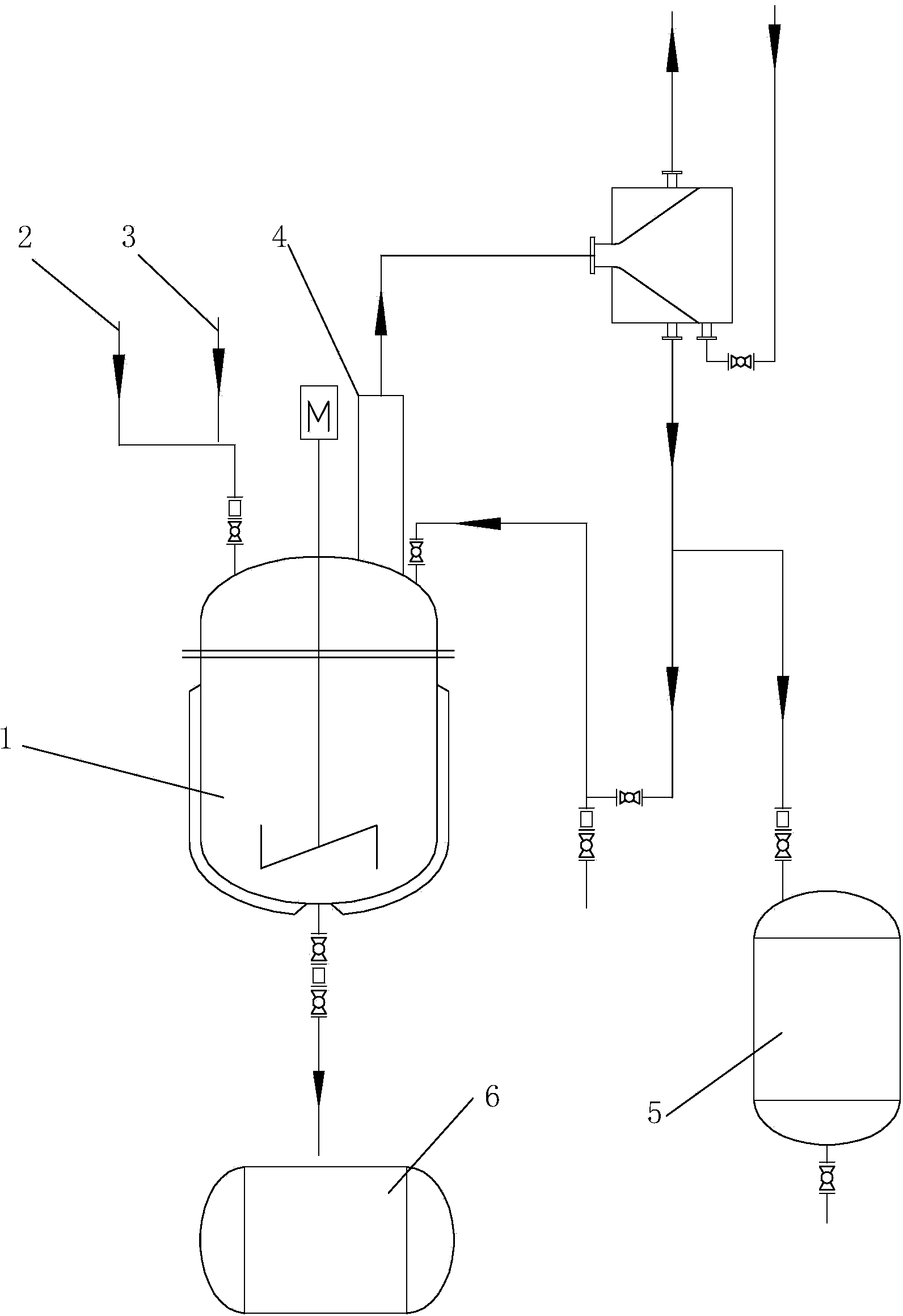
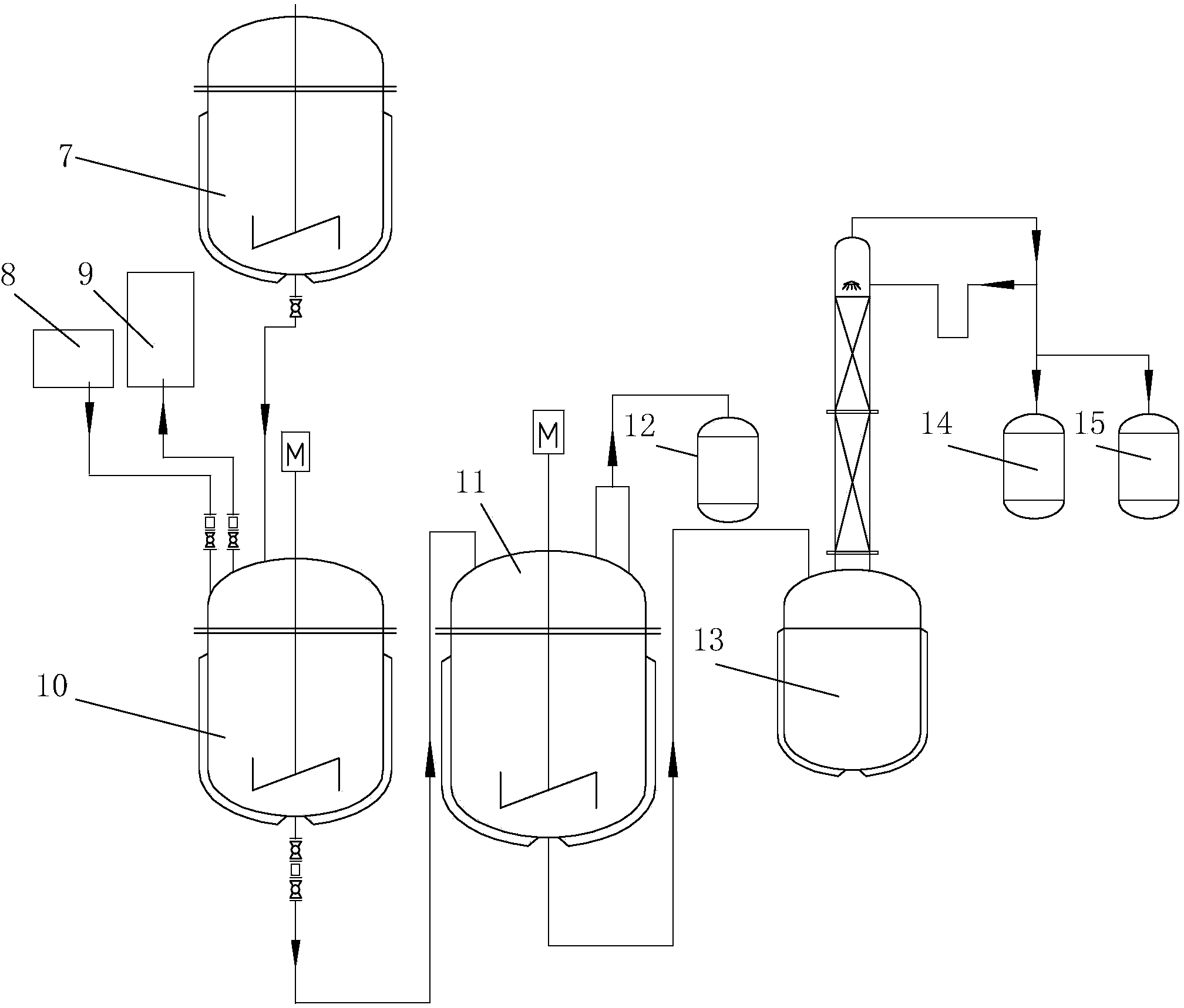
[0041] Embodiment 1: a kind of preparation method of 2-methyl-1-ethynyl-2-penten-1-alcohol, comprises the following steps:
[0042] (1) The first step reaction
[0043] (1) Calculate the amount of water according to the weight ratio of water and propionaldehyde of 2.5:1, add domestic water into the alkenal reaction kettle, and then add NaOH and K respectively according to the amount of water 2 CO 3 Configured as 0.1% NaOH aqueous solution and 2.5% K 2 CO 3 Aqueous mixture.
[0044](2) Open the jacketed circulating water of the reactor (the water is at normal temperature) for heat preservation. The aldol condensation reaction is an exothermic reaction. The circulating water at normal temperature is passed into the reactor jacket to prevent the temperature in the reactor from overheating. The production process There is no need to measure the temperature in particular, as long as circulating water at normal temperature is passed through the reactor jacket, the technical requ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com