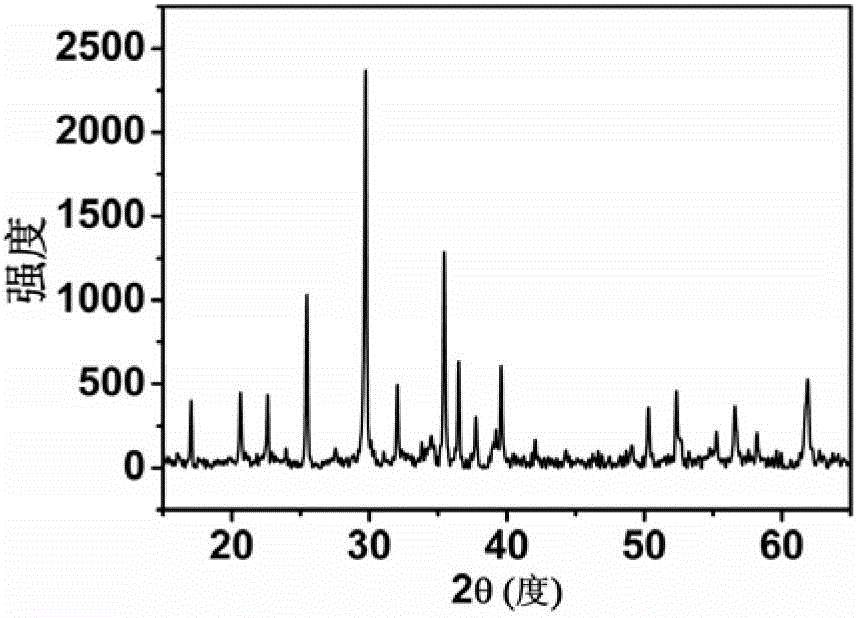
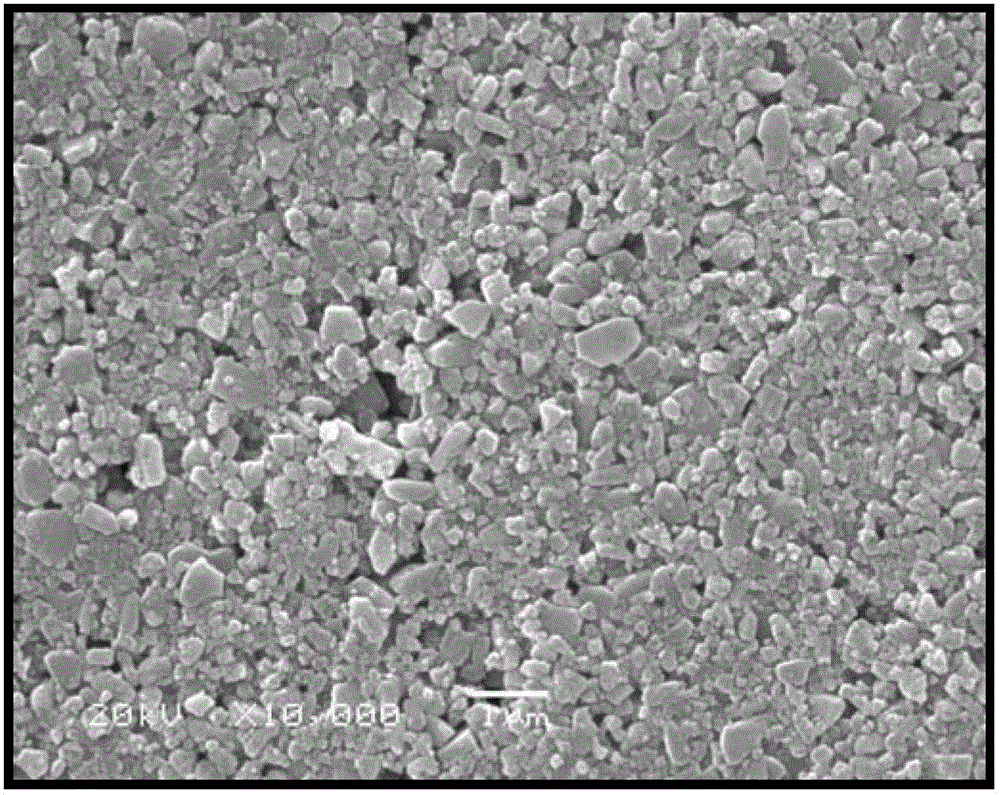
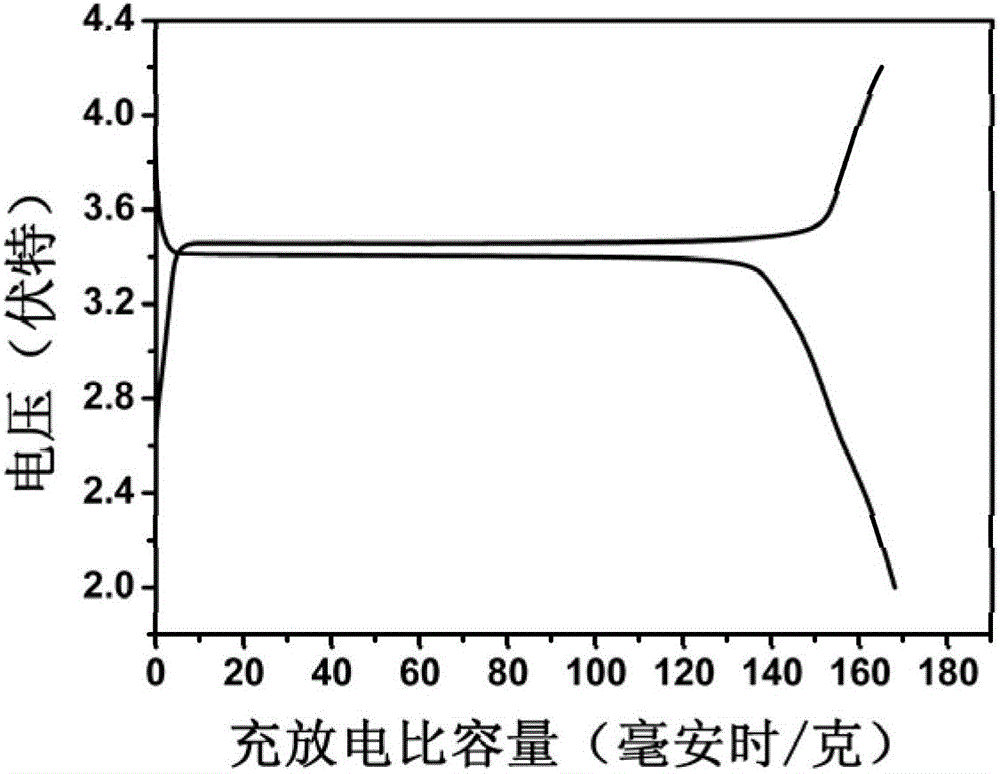
Preparation method of lithium ion battery positive pole material LiFePO4/C
A technology for lithium ion batteries and cathode materials, applied in battery electrodes, secondary batteries, circuits, etc., can solve problems such as crystallinity, morphology and particle size that cannot be balanced, and achieve excellent electrochemical performance, low reaction temperature, and reaction. short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Weigh FeSO at a molar ratio of 1:1:1 4 ·5H 2 O, H 3 PO 4 and LiOH·H 2 O. FeSO 4 ·5H 2 O and H 3 PO 4 Dissolved in ionized water, under the condition of continuously feeding argon into the above solution, add LiOH to the above solution to obtain a reaction precursor (the concentration of lithium ions is 2mol L -1 ), at this time, the pH of the reaction system is 5.0. Under the condition of continuously feeding argon into the reaction precursor, transfer it into a hydrothermal reaction kettle, and pass argon into the kettle to remove the air in the kettle. The reactor was placed in a blast drying oven, and after reacting at 190°C for 6 hours, it was taken out from the blast drying oven and cooled to 40°C. After the kettle was cooled, the reaction product was taken out and washed three times with deionized water. Finally, vacuum drying at 50°C for 12 hours to obtain LiFePO 4 powder. A certain amount of sucrose was dissolved in deionized water, and then the pre...
Embodiment 2
[0047] Weigh FeCl at a molar ratio of 1:1:4 2 , NH 4 h 2 PO 4 and Li 2 SO 4 . FeCl 2 and NH 4 h 2 PO 4 Dissolved in deionized water, under the condition of continuously passing argon gas into the above solution, add Li 2 SO 4 Obtain reaction precursor (the concentration of lithium ion is 9mol L -1 ), at this time, the pH of the reaction system is 8.0. Under the condition of continuously feeding argon into the reaction precursor, transfer it into a hydrothermal reaction kettle, and pass argon into the kettle to remove the air in the kettle. The reactor was placed in a blast drying oven, and after reacting at 190°C for 6 hours, it was taken out from the blast drying oven and cooled to room temperature. After the kettle was cooled, the reaction product was taken out and washed three times with deionized water. Finally, vacuum drying at 120°C for 1 h to obtain LiFePO 4 powder. A certain amount of glucose was dissolved in deionized water, and then the prepared LiFeP...
Embodiment 3
[0050] Weigh Fe(NO 3 ) 3 9H 2 O, H 3 PO 4 and CH 3 COOLi. Fe(NO 3 ) 3 9H 2 O and H 3 PO 4 Dissolve in deionized water, add CH 3 COOLi obtains the reaction precursor (the concentration of lithium ions is 8mol L -1 ), at this time, the pH of the reaction system is 7.0. Under the condition of continuously feeding nitrogen into the reaction precursor, it was transferred into a hydrothermal reaction kettle, and argon was passed into the kettle to remove the air in the kettle. The reactor was placed in a blast drying oven, and after reacting at 100°C for 10 h, it was taken out from the blast drying oven and cooled to room temperature. After the kettle was cooled, the reaction product was taken out and washed three times with deionized water. Finally, vacuum drying at 60°C for 12 hours to obtain LiFePO 4 powder. A certain amount of acetylene black and the prepared LiFePO 4 Powder ball milling, LiFePO 4 The mass ratio of powder to acetylene black is LiFePO 4 :C=100:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific energy | aaaaa | aaaaa |
| Specific power | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com