3,4(6)-disubstitued-1,3-benzoxazine-2-ketone compound with bactericidal acitivity
A ketone compound and benzoxazine technology, applied in the field of pesticides, can solve the problems of few studies on agricultural bactericidal activity and no reports, and achieve the effect of simple synthesis method and good bactericidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
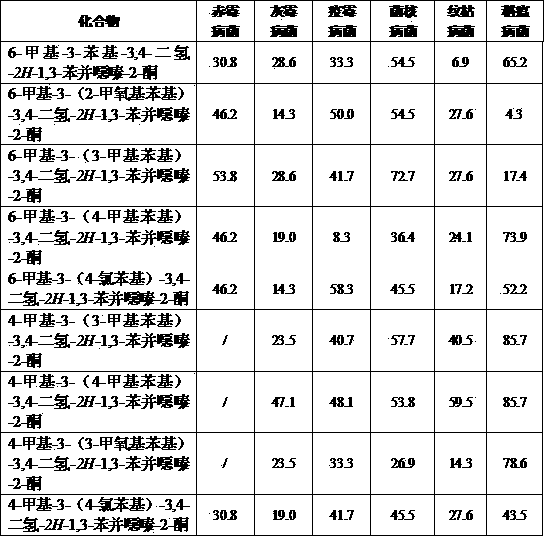
Image
Examples
Embodiment 1
[0034] Example 1: 6-Methyl-3-phenyl-3,4-2 H Synthesis of -1,3-Benzoxazin-2-one
[0035] Add 0.426g of 4-methyl-2-((anilino)methyl)phenol into a 100 mL round bottom flask, add 40 mL of solvent tetrahydrofuran, dropwise add 0.606 g of triethylamine, and slowly drop 10 mL of tetrahydrofuran solution containing 0.300 g of triphosgene was reacted at room temperature for 30 min, and then the reaction system was transferred to an oil bath and heated to 60 °C for 2 h. After the reaction was complete (TLC detection), 10 mL of ethyl acetate was added, and suction filtered. The filter cake was washed with ethyl acetate, desolvated under reduced pressure, separated and purified by column chromatography to obtain a white solid, yield: 91.0%. Melting point: 158.7-159.27°C.
[0036] 1 H NMR (CDCl 3 , 500 MHz) δ: 7.43-7.46 (m, 2H), 7.38-7.40 (m, 2H), 7.30-7.33 (m, 1H), 7.11 (d, J=10 Hz, 1H), 7.01 (d, J =10 Hz, 1H), 6.93 (s, 1H), 4.79 (s, 2H), 2.33 (s, 3H)
[0037] 13 C NMR (CDCl 3 , ...
Embodiment 2
[0039] Example 2: 6-methyl-3-(2-methoxyphenyl)-3,4-2 H Synthesis of -1,3-Benzoxazin-2-one
[0040] Add 0.365g of 4-methyl-2-((2-methoxyanilino)methyl)phenol into a 100 mL round-bottomed flask, add 40 mL of solvent tetrahydrofuran, add dropwise 0.455 g of triethylamine, and place in an ice bath Slowly add 10 mL of tetrahydrofuran solution containing 0.222 g of triphosgene dropwise, react at room temperature for 30 min, then transfer the reaction system into an oil bath and heat to 60 °C for 2 h, after the reaction is complete (TLC detection), add ethyl acetate 10 mL, suction filtered, the filter cake was rinsed with ethyl acetate, desolvated under reduced pressure, separated and purified by column chromatography to obtain a white solid, yield: 79.8%. Melting point: 182.8-184.0°C.
[0041] 1 H NMR (CDCl 3 , 500 MHz) δ: 7.35 (td, J 1 =1.5 Hz, J 2 =8 Hz, 1H), 7.31 (dd, J 1 =1.5 Hz, J 2=8 Hz , 7.09 (d, J=5 Hz, 1H), 6.99-7.03 (m, 3H), 6.89 (s, 1H), 4.71 (d, J=3.5 Hz, 2H), ...
Embodiment 3
[0044] Example 3: 6-methyl-3-(3-methylphenyl)-3,4-2 H Synthesis of -1,3-Benzoxazin-2-one
[0045] Add 0.341 g of 4-methyl-2-((3-methylanilino)methyl)phenol into a 100 mL round-bottomed flask, add 40 mL of solvent tetrahydrofuran, and dropwise add 0.455 g of triethylamine. , slowly drop 10 mL of tetrahydrofuran solution containing 0.222 g of triphosgene, react at room temperature for 30 min, then transfer the reaction system into an oil bath and heat to 60 °C for 2 h, after the reaction is complete (TLC detection), add 10 mL of ethyl acetate, After suction filtration, the filter cake was rinsed with ethyl acetate, desolvated under reduced pressure, separated and purified by column chromatography to obtain a white solid with a yield of 89.5%. Melting point: 133.2-134.3°C.
[0046] 1 H NMR (CDCl 3 , 500 MHz) δ: 7.26 (dd, J 1 =8.5 Hz, J 2 =15.5 Hz, 4H), 7.11 (d, J=8.5 Hz, 1H), 7.0 (d, J=8 Hz, 1H), 6.92 (s, 1H), 4.77 (s, 2H), 2.37 (s, 3H ), 2.33 (s, 3H).
[0047] 13 C NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com