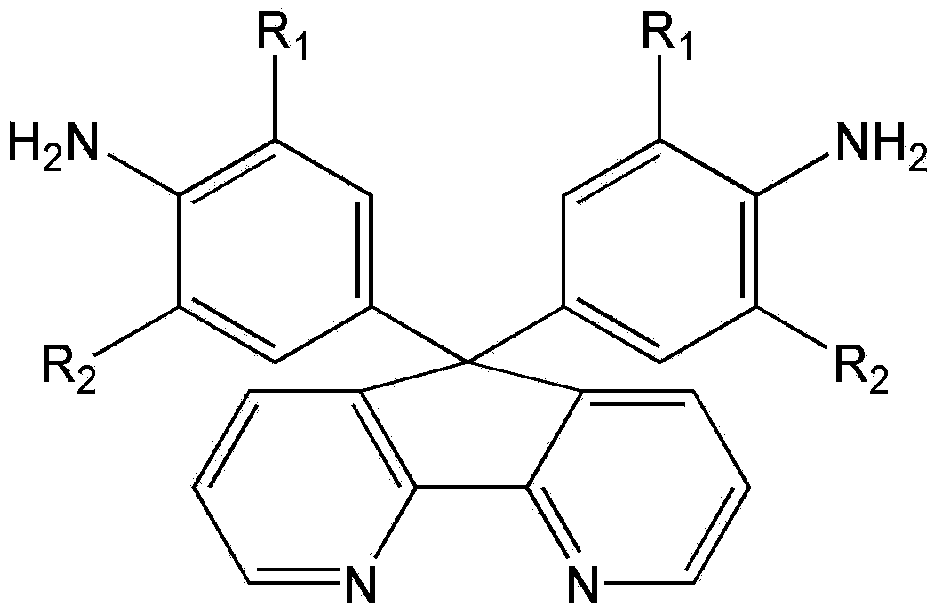
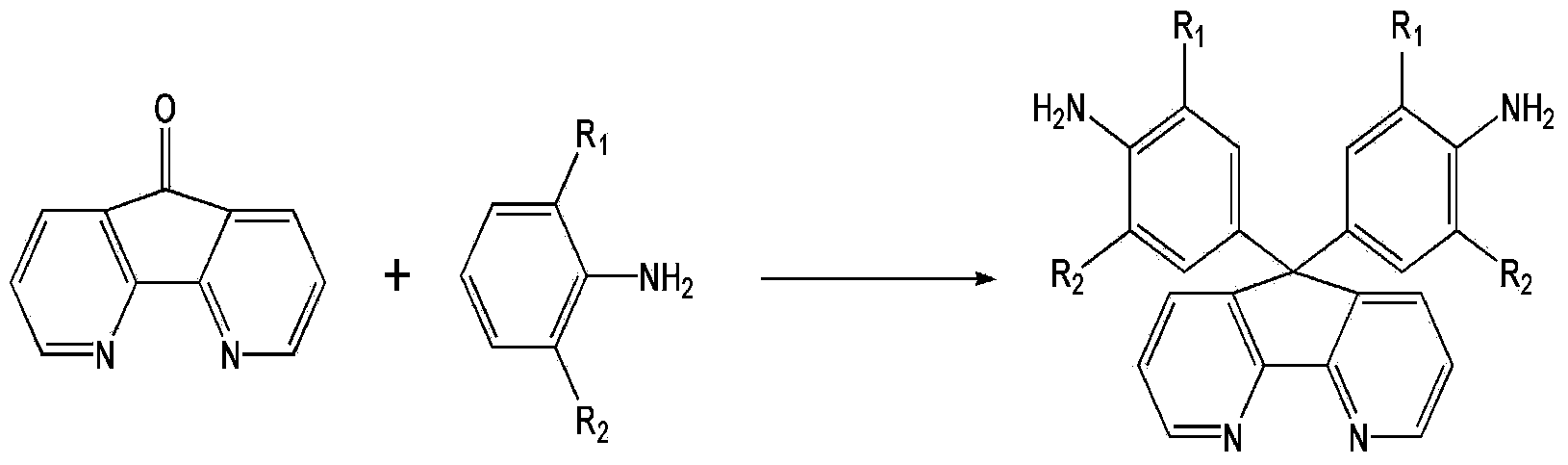
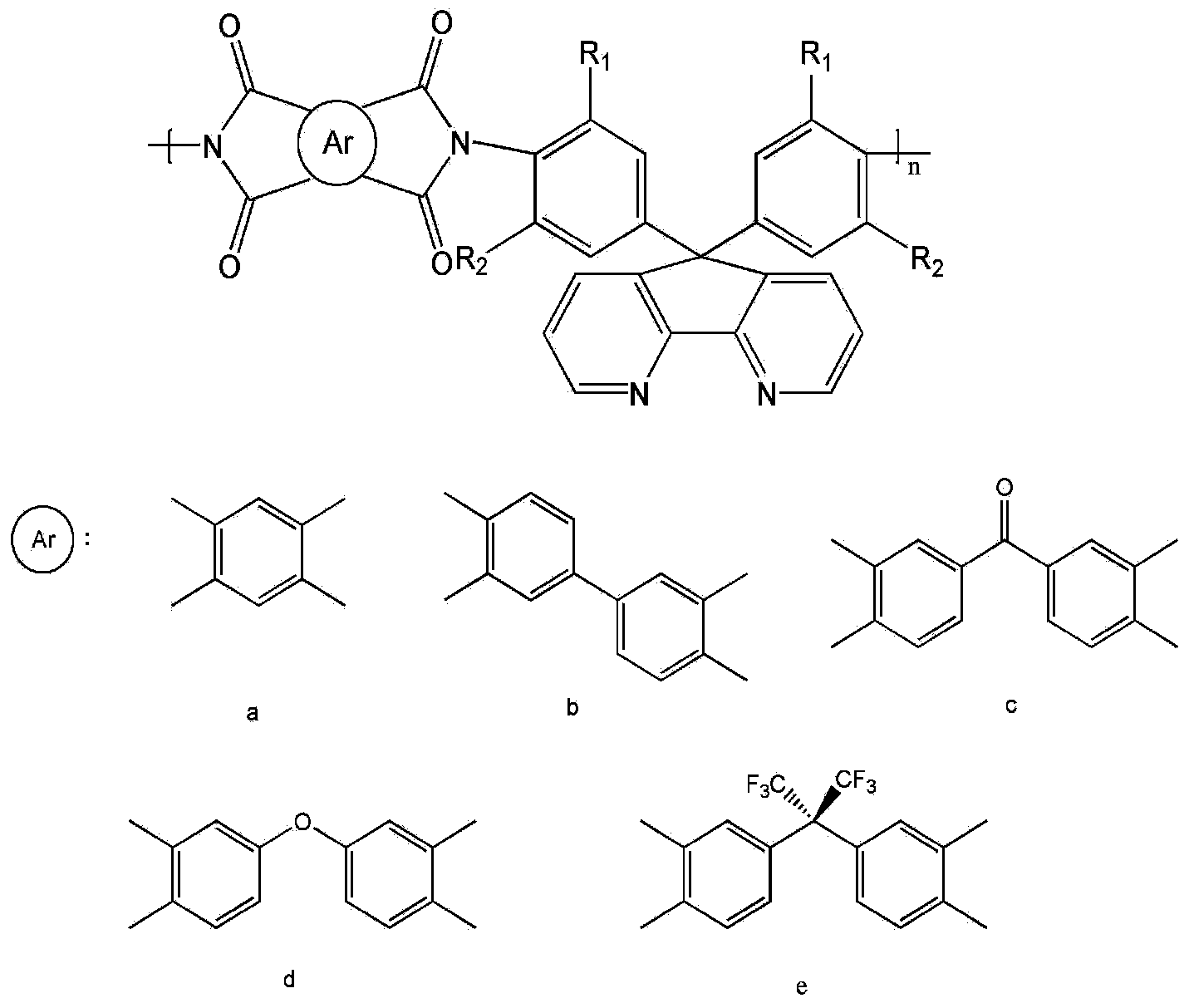
4,5-diazafluorene-containing aromatic diamine as well as preparation method and application thereof
A technology of aromatic diamine and diazofluorene, which is applied in the field of aromatic diamine containing 4,5-diazofluorene and its preparation, can solve the problems of high melting temperature or softening temperature and poor solubility of polyimide, and achieve excellent Heat resistance and moist heat oxidation resistance, excellent film-forming performance, and high light transmission performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, the preparation method of 9,9-bis(4-aminophenyl)-4,5-diazofluorene of the present invention:
[0033] ① Add 100-150 parts (parts by weight) of aniline to a three-neck round bottom flask, add 30-50 parts of methanesulfonic acid or trifluoromethanesulfonic acid under stirring, and then add 30-50 parts of 4,5- Diazofen-9-one, stir well. In pass N 2 Under certain conditions, heat up to 50-170°C and react for 5-20 hours. When cooled below 80°C, neutralize with NaOH solution, filter, wash with water, and purify by recrystallization to obtain 9,9-bis(4-aminophenyl)-4,5-diazofluorene. Melting point: >300°C. FT-IR(KBr):3454-3331cm -1 (N–H stretch),3066~3004cm -1 (C–H stretch),1510cm -1 (C=N stretch). 1 H NMR (400MHz, DMSO-d 6 ,δ,ppm):5.03(s,4H,-NH 2 ), 6.42(d, J=8.4Hz, 2H), 6.75(d, J=8.4Hz, 2H), 7.35(dd, J=4.8, 4.8Hz, 2H,), 7.84(dd, J=1.6, 1.6 Hz,2H),8.62(dd,J=1.6,1.6Hz,2H). 13 C NMR (100MHz, DMSO-d 6 ,δ,ppm):59.8,114.1,123.9,128.5,131.0,134.2,147.5,148...
Embodiment 2
[0034] Embodiment 2, the preparation method of 9,9-bis(3-methyl-4-aminophenyl)-4,5-diazofluorene of the present invention:
[0035] ①Add 100-150 parts (by weight) of o-methylaniline to a three-necked round bottom flask, add 30-50 parts of methanesulfonic acid or trifluoromethanesulfonic acid under stirring, and then add 30-50 parts of 4 , 5-diazofluoren-9-one, stir well. In pass N 2 Under certain conditions, heat up to 50-170°C and react for 5-20 hours. When cooled to below 80°C, pour it into NaOH solution, stir overnight, filter, wash with water, and purify by recrystallization to obtain 9,9-bis(3-methyl-4-aminophenyl)-4, 5-diazofluorene. Melting point: 312-314°C. 1 H NMR (400MHz, Acetone-d 6 ,δ,ppm):2.0(m,6H),4.29(s,4H,-NH 2 ),6.54(dd,J=1.6,1.6Hz,2H),6.76(t,J=8.4,18Hz,4H),7.28(m,2H,),7.82(m,2H),8.61(m,2H) . 13 C NMR (100MHz, Acetone-d 6 ,δ,ppm):17.7,61.0,115.2,122.4,123.9,127.2,130.4,133.6,134.5,146.1,148.5,150.2,158.7.(EI-m / z):[M+H] + calcd for C 25 h 22 N 4 ,378...
Embodiment 3
[0036] Example 3, the preparation method of 9,9-bis(4-amino-3,5-difluorophenyl)-4,5-diazofluorene of the present invention:
[0037] ①Add 100-150 parts (parts by weight) of 2,6-difluoroaniline into a three-neck round bottom flask, add 30-50 parts of methanesulfonic acid or trifluoromethanesulfonic acid under stirring, and then add 30- 50 parts of 4,5-diazofluoren-9-one, fully stirred. In pass N 2 Under certain conditions, heat up to 50-170°C and react for 5-20 hours. When cooled to below 80°C, pour it into NaOH solution, stir overnight, filter, wash with water, and purify by recrystallization to obtain 9,9-bis(4-amino-3,5-difluorophenyl)- 4,5-diazofluorene. Melting point: 290-292°C. 1 H NMR (400MHz, Acetone-d 6 ,δ,ppm):4.79(s,4H,-NH 2 ),6.70(dd,J=2.4,2.0Hz,4H),7.41(dd,J=4.8,4.8Hz,2H),8.03(dd,J=1.6,1.2Hz,2H,),8.71(d,J =3.6Hz,2H). 13 C NMR (100MHz, Acetone-d 6 ,δ,ppm):60.3,111.4(J C-F =6.2,8.2Hz),124.5,125.5(J C-F =165Hz), 131.9(J C-F =78Hz), 134.5, 146.2, 148.0, 151...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com