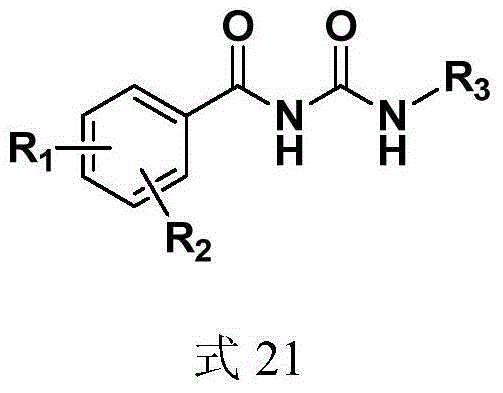
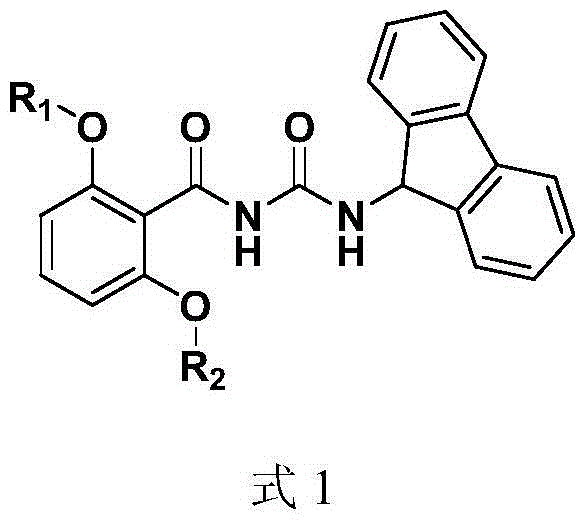
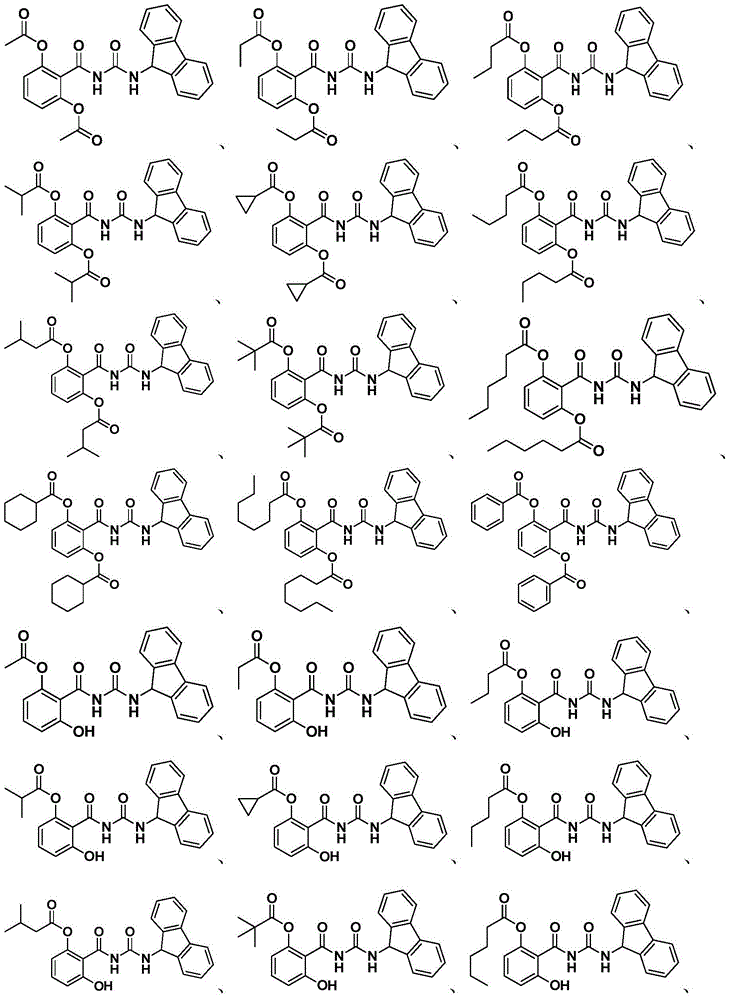
Anti-hepatitis C virus compound, preparation method and application thereof
A compound and composition technology, applied in the field of medicinal chemistry and pharmacotherapeutics, can solve the problems of poor solubility, affecting bioavailability and drug efficacy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Synthesis of 2,6-dibenzyloxybenzonitrile (compound shown in formula 4)
[0125] Dissolve 0.5 g of sodium benzyl alcohol in 10 ml of dimethyl sulfoxide, and add 2,6-difluorobenzonitrile under stirring. After rapidly raising the temperature to 115°C, stirring was continued for 8 hours. After cooling to room temperature, 100 ml of ice water was poured into the reaction liquid, and the stirring was continued for 30 minutes. A large amount of solid precipitated out, which was filtered by suction, washed with water, and dried to obtain 2,6-dibenzyloxybenzonitrile as a white solid.
[0126] 1 H-NMR (400Mz, DMSO-d6) δ: 5.19 (4H, s), 6.60 (2H, d), 7.30-7.35 (3H, m), 7.38 (4H, t), 7.45 (4H, d).
Embodiment 2
[0128] Synthesis of 2,6-dibenzyloxybenzamide (compound shown in formula 5)
[0129] Dissolve 0.4 gram of 2,6-dibenzyloxybenzonitrile (compound shown in formula 4) in 7 milliliters of benzyl alcohol, and add 0.6 gram of potassium hydroxide, 0.6 gram of n-tetrabutylammonium bromide and 0.1 milliliter of water, 140 Stir at °C for 24 hours. After the reaction, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography to obtain 2,6-dibenzyloxybenzamide as a white solid.
[0130] 1 H-NMR (400Mz, DMSO-d6) δ: 5.15 (4H, s), 6.60 (2H, d), 7.18-7.48 (12H, m), EI-MS m / z333.2 (M + ), 91.1 (100%).
Embodiment 3
[0132] Synthesis of 1-(fluoren-9-yl)-3-(2,6-dibenzyloxybenzoyl)urea (compound represented by formula 8)
[0133] Dissolve 0.5 g of triphosgene in 3 ml of 1,2-dichloroethane, and stir in an ice bath until dissolved. Dissolve 0.25 g of 9-fluorenamine in 10 ml of 1,2-dichloroethane, and slowly add it dropwise to the reaction solution containing triphosgene. The reaction solution is white and turbid. Continue to stir in the ice bath for 0.5 hours and then heat up to 25 °C for 1 hour, and then heated to reflux until the reaction solution was colorless and transparent, and 1 ml of triethylamine was added to the reaction solution to continue the reflux reaction for 0.5 hours. The solvent was evaporated under reduced pressure, and 10 ml of toluene was added for filtration. 0.35 g of 2,6-dibenzyloxybenzamide (compound represented by formula 5) was added to the filtrate, and refluxed for 12 hours. The solvent was distilled off under reduced pressure, and the residue was purified by si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com