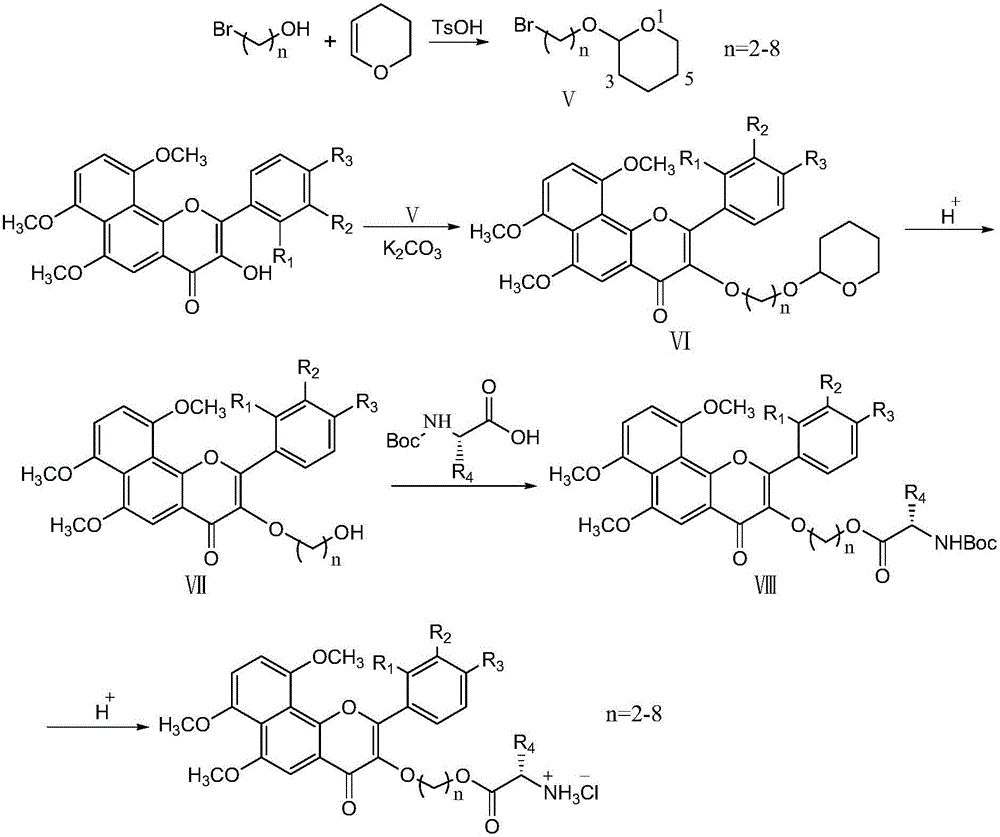
Water-soluble alpha-naphthoflavone alcohol derivative as well as preparation method and application thereof
A technology of naphthoflavone alcohol and naphthoflavone alcohol hydroxy alkyl ether is applied in the field of water-soluble α-naphthoflavone alcohol derivatives and preparation thereof, and can solve the problems of limited application, poor solubility and the like, and achieves simple operation, easy availability of raw materials, The effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
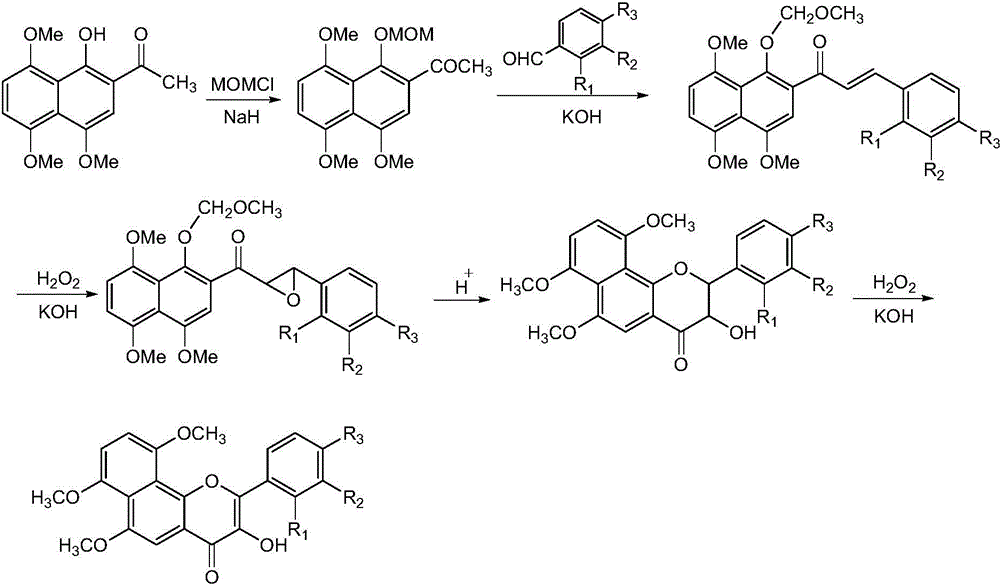
[0034] This embodiment relates to a preparation method of 6,7,10-trimethoxy-α-naphthalene flavonol (II-1) with structural formula (II), such as figure 1 shown, including the following steps:
[0035] Step 1: Dissolve 2-acetyl-4,5,8-trimethoxy-1-naphthol in anhydrous N,N'-dimethylformamide, add 2.0 equivalents of sodium hydride and 1.6 equivalents Chloromethyl methyl ether, after stirring at room temperature for 3h, adding saturated sodium bicarbonate solution to quench the reaction, CH 2 Cl 2 Extract and combine the organic layers. The organic layer was washed with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate, concentrated, and silica gel column chromatography to obtain 4,5,8-trimethoxy-2-acetyl-1-naphthol methoxymethyl ether, It was a yellow solid with a yield of 92%. 1H NMR (300MHz, CDCl 3 ): δ6.99(s, 1H, H-C(2)), 6.93(d, J=8.7Hz, 1H), 6.88(d, J=8.7Hz, 1H), 5.01(s, 2H, OCH 2 O), 3.96(s, 3H, OCH 3 ), 3.93 (s, 3H, OCH 3 ), 3.90 (s, 3H, OC...
Embodiment 2
[0041] This embodiment relates to a preparation method of 2'-fluoro-6,7,10-trimethoxy-α-naphthalene flavonol (II-2) with structural formula (II), such as figure 1 shown, including the following steps:
[0042] The steps of this example are the same as the steps of Example 1, and in step 2, 2-fluorobenzaldehyde is used instead of benzaldehyde. The product was yellow crystals with a total yield of 15%. 1 H NMR (400MHz, d 6 -DMSO): δ9.49(s, 1H, OH), 7.86(t, J=6.9Hz, 1H), 7.63(m, 1H), 7.43(m, 2H), 7.33(s, 1H, H-C(5 )), 7.27(d, J=8.6Hz, 1H), 7.22(d, J=8.6Hz, 1H), 3.96(s, 3H, OCH 3 ), 3.84(s, 6H, OCH 3 ).
Embodiment 3
[0044] This embodiment relates to a preparation method of 3'-fluoro-6,7,10-trimethoxy-α-naphthalene flavonol (II-3) with structural formula (II), such as figure 1 shown, including the following steps:
[0045] The steps of this example are the same as those of Example 1, and in step 2, 3-fluorobenzaldehyde is used instead of benzaldehyde. The product was yellow crystals with a total yield of 13%. 1 H NMR (400MHz, d 6 -DMSO): δ10.03(s, 1H, OH), 8.33(d, J=7.9Hz, 1H, H-C(2')), 8.23(d, J=11.4Hz, 1H, H-C(6')) , 7.68(dd, J=14.7, 7.9Hz, 1H, H-C(5')), 7.37(m, 1H, H-C(4')), 7.31(s, 2H), 7.30(s, 1H, H-C(5') )), 4.09(s, 3H, OCH 3 ), 3.96 (s, 3H, OCH 3 ), 3.85(s, 3H, OCH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com