Ligand compound containing pyridyl group, and catalyst containing ligand compound and application thereof
A ligand compound and compound technology, which is applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, hydrocarbons, etc., can solve cumbersome preparation steps, high cost, complex ligand structure, etc. problem, to achieve the effect of simple synthesis steps, low cost, and simple ligand structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
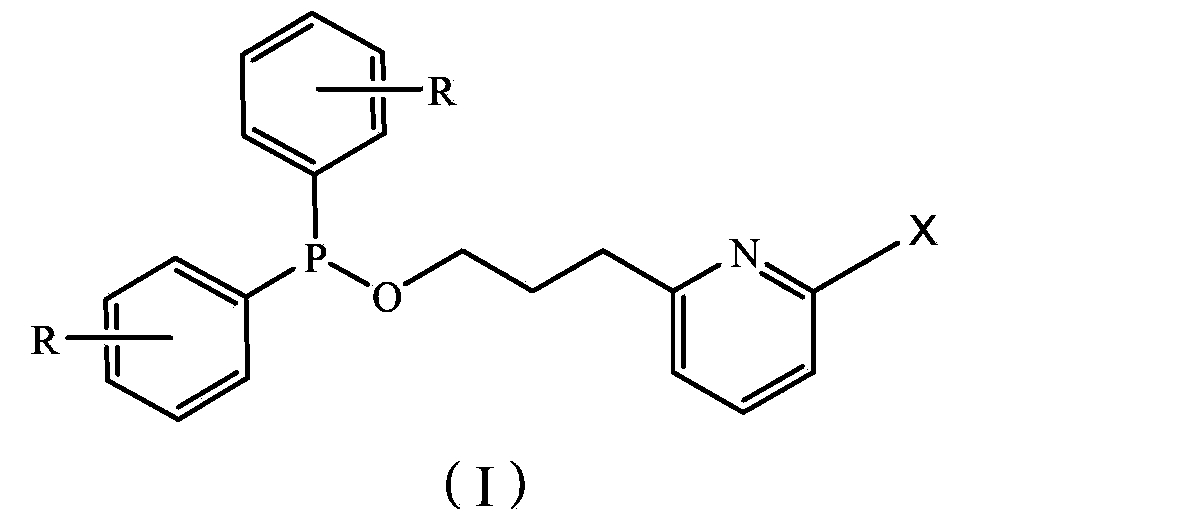
[0028] Synthesis of Ligand A, Ligand A is the ligand shown in formula III, wherein R=H.
[0029] The ligand preparation method is as follows: under the protection of nitrogen, add 0.01mol diphenylphosphorous chloride, 100mL anhydrous ether and 1mL triethylamine to a round bottom flask, stir, cool to 0 °C in an ice bath, add 0.01mol 2-propane Alcohol-based pyridine, stirred and reacted for about 1 hour, filtered, and the filtrate was distilled under reduced pressure (10 mm Hg) with a vacuum water pump to obtain a white oil, which was purified by column chromatography (eluent was petroleum ether and dichloromethane), The target product ligand A was obtained. Yield 65%. 1 H-NMR (δ, ppm, CDCl 3 , TMS): 7.1~8.4(m, 14H, Ar-H and Py-H), 3.5(t, 2H, CH 2 -O), 2.8(t, 2H, CH 2 ), 1.8 (m, 2H, CH 2 ).
Embodiment 2
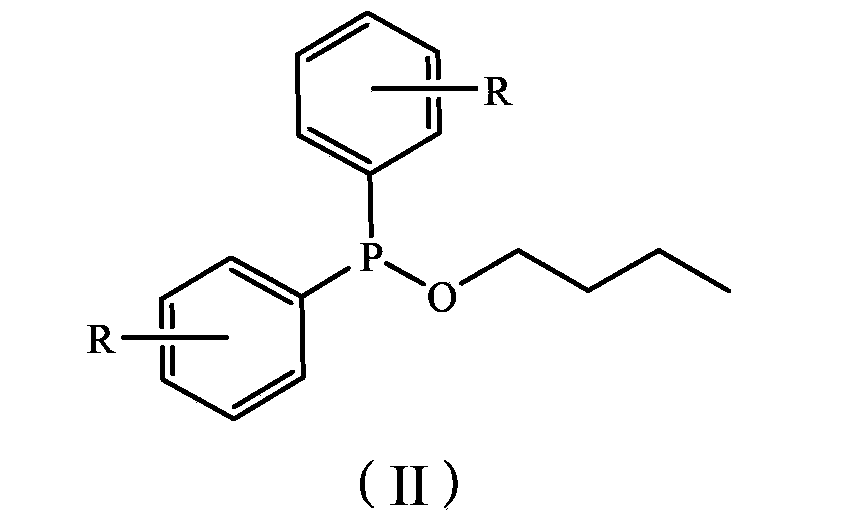
[0031] Synthesis of Ligand B, Ligand B is a ligand represented by formula III, wherein R=2-F.
[0032] The preparation method is the same as Ligand Synthesis Example 1, except that diphenylphosphorus chloride is replaced by di(o-fluorophenyl)phosphorus chloride, and other conditions remain unchanged. Yield 59%. 1 H-NMR (δ, ppm, CDCl 3 , TMS): 7.0~8.4(m, 12H, Ar-H and Py-H), 3.5(t, 2H, CH 2 -O), 2.8(t, 2H, CH 2 ), 1.8 (m, 2H, CH 2 ).
Embodiment 3
[0034] Synthesis of ligand C, ligand C is a ligand shown in formula IV, wherein R=H.
[0035] The preparation method is the same as Ligand Synthesis Example 1, the difference is that 2-propanol pyridine is replaced by 2,6-dipropanol pyridine, the amount of diphenyl phosphorus chloride is changed from 0.01mol to 0.02mol, other conditions constant. Yield 55%. 1 H-NMR (δ, ppm, CDCl 3 , TMS): 7.1~7.6(m, 23H, Ar-H and Py-H), 3.6(t, 4H, CH 2 -O), 2.9(t, 4H, CH 2 ), 1.8(m, 4H, CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com