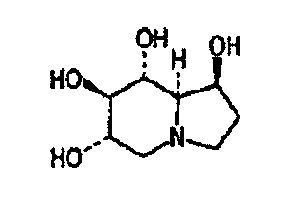
Application of castanapetmine in preparation of anti-AIDS drugs
A technology of anti-AIDS and drugs, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of chestnut sourine
[0025] Take 500g of dried leaf powder of Dahuabudou, put it in an extraction kettle, add methanol as an entrainer at a solid-to-liquid volume ratio of 5:2, and feed in liquid CO at a pressure of 22MPa and a temperature of 38°C 2 , CO 2 The flow rate is 4.5ml / g crude drug / min, extract for 120min, analyze at a pressure of 7MPa and a temperature of 32°C, collect the extract, add 15g of diatomaceous earth to stir for decolorization, filter to obtain a decolorization solution, and concentrate to a relative density of 1.12, above eluted with petroleum ether-ethyl acetate 3:14 to obtain the crude sourine of chestnut seed; with methanol at a volume ratio of 19:38:45 -Acetonitrile-water is the mobile phase, the crude product is dissolved in the mobile phase, injected into the preparative high-performance liquid chromatograph, the flow rate is controlled to be 20ml / min, the target fraction is collected, prepared continuously, and freeze-drie...
Embodiment 2
[0045] Tablet: 40g of chestnut soycine, 135g of starch, 25g of chenodeoxycholate, granulated and compressed into 1000 tablets. Dosage: Adults take 1 tablet at a time, 2 times a day.
Embodiment 3
[0047] Capsules: 40g of chestnut sourine, 135g of starch, 25g of chitosan, mixed well, made into 1000 capsules. Dosage: Adults take 1 capsule at a time, 2 times a day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com