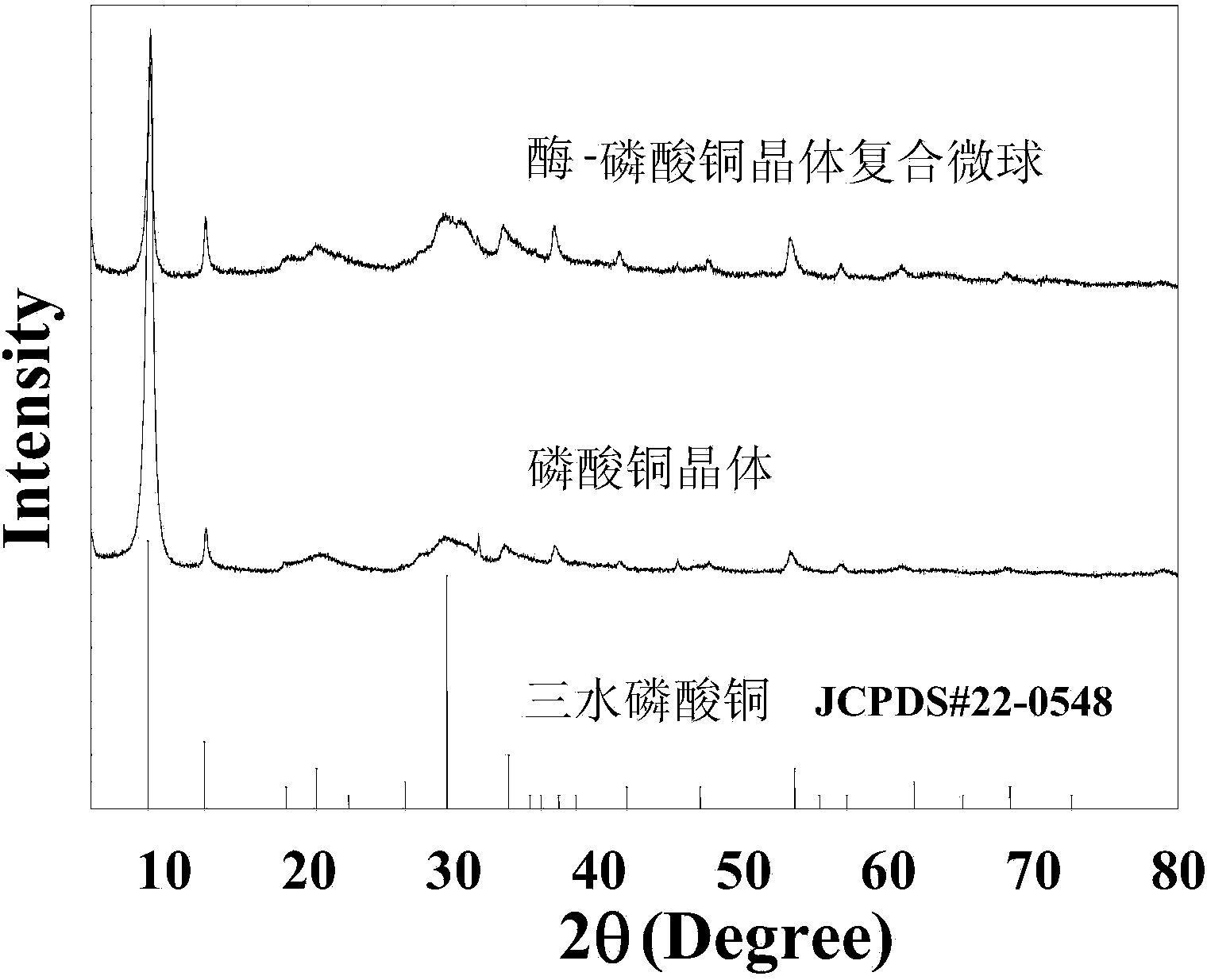
Enzyme-inorganic crystal compound microsphere and preparation method thereof
A technology of inorganic crystals and composite microspheres, which is applied in the direction of immobilization on or in the inorganic carrier, can solve the problems of high preparation cost, complicated preparation method, low yield of immobilized enzyme activity, etc., and achieve simple operation and low cost , the effect of stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, the preparation of candida antarctica lipase B (CALB)-copper phosphate crystal composite microsphere
[0045] 1. Prepare CALB PBS buffer (pH=7.4) with an enzyme concentration of 0.5 mg / mL and 200 mM copper sulfate pentahydrate aqueous solution.
[0046] 2. Take 5mL of the enzyme solution in step 1 into a glass vial, add 0.05mL of the copper sulfate solution in step 1, mix well, and put it in a 25°C incubator for 24h.
[0047] 3. After filtering the mixture obtained in step 2, the precipitate was collected, washed 3 times with deionized water, filtered again to collect the precipitate, and freeze-dried for 5 hours to obtain copper phosphate crystal-Candida antarctica lipase B composite microspheres.
[0048] The scanning electron micrograph of the composite microsphere prepared in this embodiment is as follows figure 2 shown by figure 2 It can be seen that the particle size of the composite microsphere is about 10 μm. The enzyme loading in the composit...
Embodiment 2-4
[0049] Embodiment 2-4, preparation of glucose dehydrogenase-copper phosphate crystal composite microspheres, cellobiose dehydrogenase-copper phosphate crystal composite microspheres and uricase-copper phosphate crystal composite microspheres
[0050]The Candida antarctica lipase B (CALB) in the preparation step 1 of Example 1 was replaced with glucose dehydrogenase, cellobiose dehydrogenase and uricase respectively, and other operations were the same.
[0051] The scanning electron microscope image of glucose dehydrogenase-copper phosphate crystal composite microspheres is as follows image 3 shown by image 3 It can be seen that the particle size of the composite microsphere is about 10 μm. The enzyme load in the glucose dehydrogenase-copper phosphate crystal composite microsphere is 5%.
[0052] Scanning electron microscope image of cellobiose dehydrogenase-copper phosphate crystal composite microspheres Figure 4 shown by Figure 4 It can be seen that the particle size ...
Embodiment 5
[0054] Embodiment 5, glucose oxidase (GO X )-horseradish peroxidase (HRP)-copper phosphate crystal composite microspheres
[0055] 1. Prepare the PBS buffer solution (pH=7.4) of glucose oxidase (GOx) and horseradish peroxidase (HRP) with a total concentration of 0.5 mg / mL respectively (GO X and HRP concentrations are 0.25mg / mL) and 200mM copper sulfate pentahydrate aqueous solution.
[0056] 2. Take 5mL of the enzyme solution in step 1 into a glass vial, add 0.05mL of the copper sulfate solution in step 1, mix well, and place it in a 25°C incubator for 24h.
[0057] 3. Collect the precipitate after filtering the mixture obtained in step 2, wash with deionized water 3 times, filter again to collect the precipitate, freeze-dry for 5 hours to obtain glucose oxidase (GOx)-horseradish peroxidase (HRP)-copper phosphate Crystalline composite microspheres.
[0058] The scanning electron microscope picture of the composite microsphere prepared in this embodiment is as follows Figu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com