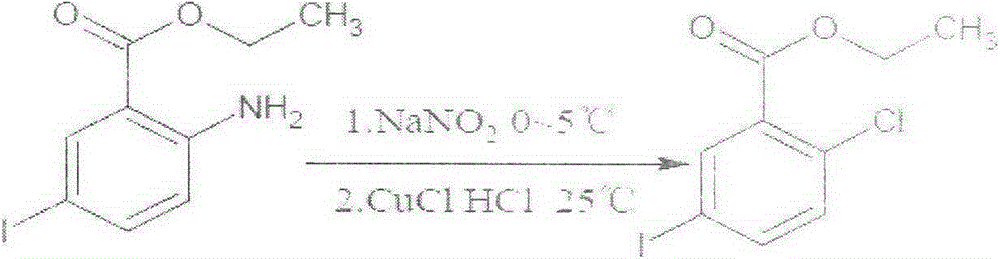
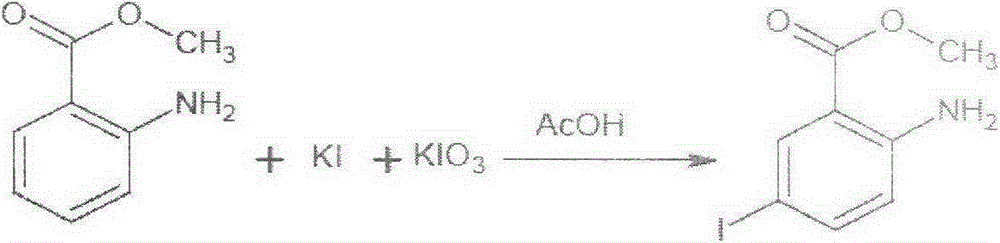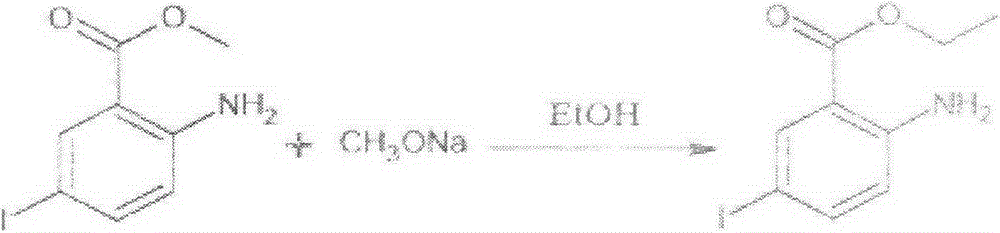Preparation method of 2-chloro-5-iodobenzoic acid
A technology of iodobenzoic acid and ethyl iodobenzoate, applied in the field of biochemistry, can solve the problems of low yield, complex production method, high production cost, etc., and achieve the effects of high product yield, reasonable structure, and improved production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] 1) Preparation of ethyl 2-amino-5-iodobenzoate
[0023] Add 450.8g of water, 185.84g of potassium iodide, and 106.64g of potassium iodate into a 2000ml three-neck flask, stir to dissolve, then add 230g of methyl anthranilate, add 100.99g of dichloromethane and 147g of glacial acetic acid dropwise at room temperature and mix Liquid, control the dropwise addition for 1h (the heat will be released during the dropping process, control the dropping temperature below 45°C), after the dropwise addition is completed, stir for 0.5h, heat up to 5°C and keep for 3h, after the reaction is complete, cool to below 25°C, Add 404g of dichloromethane in batches, stir until the brown color of the system fades; let stand to separate layers, collect the organic layer, and distill several layers of atmospheric pressure to a system temperature above 80°C (about 300ml of solvent is evaporated), and cool the remaining materials to 30°C. Below ℃, add 800g of absolute ethanol and stir until diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com