Engineering bacterium and method for preparing tert-butyl (3R, 5R) 6-cyan-3, 5-dyhydroxyl hexanoate
A technology of tert-butyl hydroxycaproate and engineering bacteria, which is applied in the field of preparing 6-cyano-3 and engineering bacteria, can solve the problems of increasing difficulty, increasing the difficulty of post-processing, increasing production cost and the like, and achieves a reduction in production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
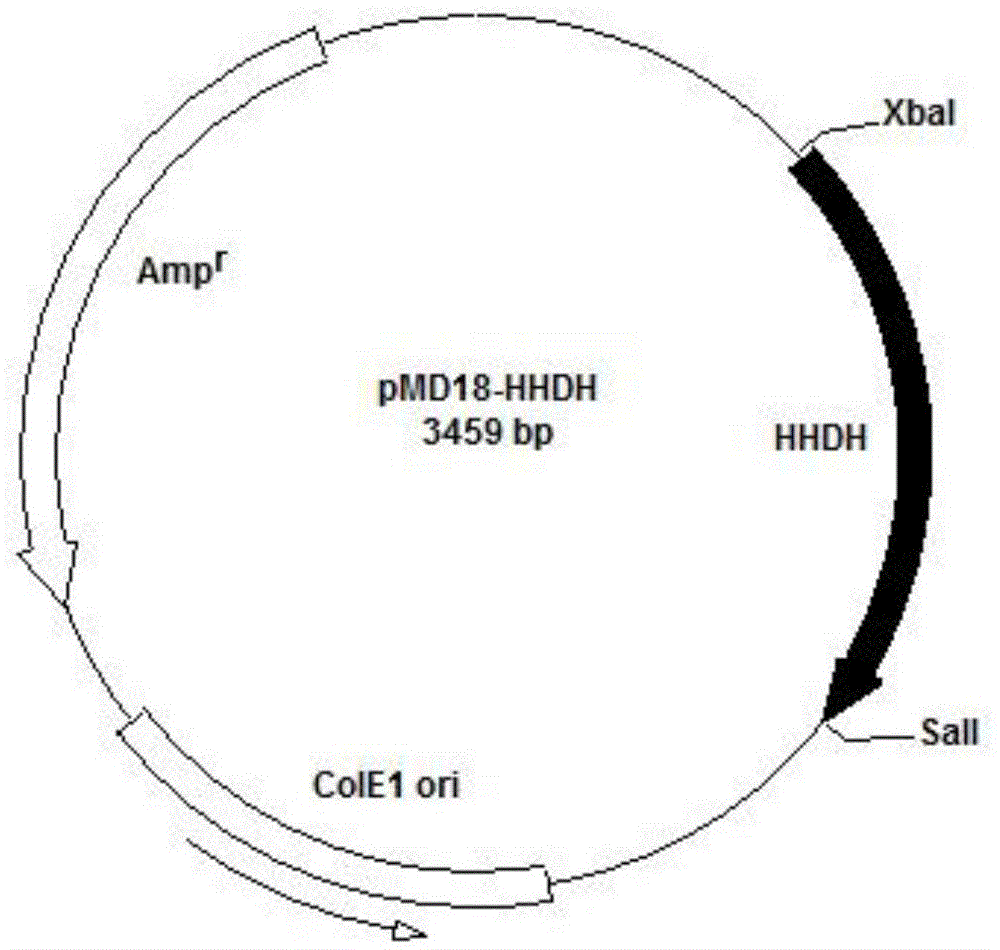
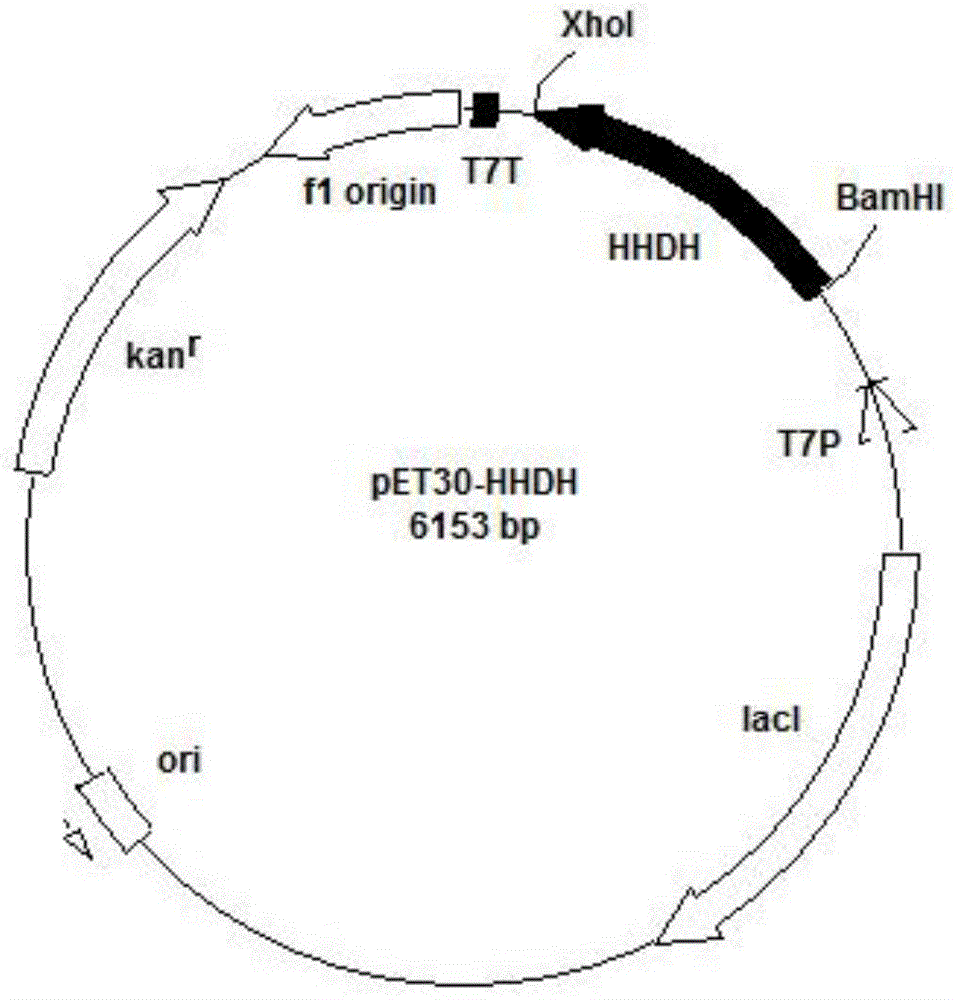
[0049] Example 1 Construction of plasmid pET30-HHDH
[0050] The hhdh gene was cloned with primers F_HHDH / R_HHDH to obtain a hhdh gene with a length of 765bp. Nucleic acid electrophoresis to verify gene size, such as figure 2 .
[0051] The sequence of primer F_HHDH is:
[0052] 5'-CGCGGATCCATGTCAACCGCAATTGTAAC-3';
[0053] The sequence of primer R_HHDH is:
[0054] 5'-CCGCTCGAGCTACTCTGGCATACCAGG-3'.
[0055] The hhdh gene sequence is as follows:
[0056] ATGTCAACCGCAATTGTAACAAACGTTAAGCATTTTGGGGGAATGGGGTCTGCACTTCGTCTCTCGGAAGCAGGACATACAGTGGCTTGCCACGATGAAAGCTTCAAACACCAAGACGAACTTGAAGCCTTTGCCGAAACCTATCCACAACTCATCCCAATGTCGGAACAAGAACCAGCGGAACTCATCGAGGCAGTTACCTCCGCTCTCGGTCACGTTGATGTACTTGTGAGCAACGACATCGCTCCGGTCGAGTGGCGCCCAATCGATAAATACGCTGTAGAGGACTATCGCGATACTGTCGAGGCGCTCCAAATTAAGCCATTTGCACTGGTCAACGCCGTTGCAAGTCAAATGAAGAAGCGCAAAAGCGGACATATTATCTTTATTACCTCTGCTGCTCCAGTTGGGCCTTGGAAGGAACTTTCTACCTACTCGTCAGCCCGTGCAGGTGCATCTGCTTTGGCAAATGCCCTTTCGAAGGAACTCGGTGAATACAACATTCCGGTGTTCGCAATCGCTCCA...
Embodiment 2
[0058] Example 2 Construction and Induced Expression of Genetically Engineered Bacteria
[0059] The expression host Escherichia coli BL21(DE3) was transformed with the plasmid pET30-HHDH constructed in Example 1. Use primers F_HHDH / R_HHDH for colony PCR to verify transformed recombinants. The verified genetically engineered bacteria is EcoH. EcoH was inoculated into the fermentation medium and cultured with constant temperature shaking for 16 hours. The culture conditions were 35° C. and 180 rpm. When the cell concentration grows to OD 600 When =0.8, add 0.4mM IPTG (final concentration) and induce at 16°C for 20h.
Embodiment 3
[0060] Example 3 Construction and Induced Expression of Genetically Engineered Bacteria
[0061] The expression host Escherichia coli BL21(DE3) was transformed with the plasmid pET30-HHDH constructed in Example 1. Use primers F_HHDH / R_HHDH for colony PCR to verify transformed recombinants. The verified genetically engineered bacteria is EcoH. EcoH was inoculated into the fermentation medium and cultured with constant temperature shaking for 16 hours. The culture conditions were 40° C. and 220 rpm. When the cell concentration grows to OD 600 When =1.2, add 0.8mM IPTG (final concentration) and induce at 22°C for 20h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com