Improved antibody chip kit capable of quantitatively detecting multiple cell factors synchronously
A cytokine and antibody chip technology, applied in the field of biomedicine, can solve the problems of less specimen consumption, cumbersome operation, and low sensitivity, and achieve the effects of reducing background interference, increasing stability, and increasing detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Screening of the best antibody chip carrier.
[0032] Conventional antibody chips mostly use nitrocellulose membrane as a carrier. Since the nitrocellulose membrane has a multi-layer structure, it is difficult to wash the chip, resulting in large fluctuations in the results of the chip. At the same time, because nitrocellulose membrane chips are not easy to operate on a large scale, the use of large-scale clinical samples is not yet common. Different manufacturers use different methods to immobilize nitrocellulose membranes on the surface of glass slides. Among them are Whatman's SS slides, which fix 16 cells containing nitrocellulose membranes on one glass slide. In addition, Gentel uses processed PATH slides were produced by coating the slide surface with nitrocellulose material. In addition, in order to coat the antibody on the surface of the slide, we screened the carriers activated by different methods. Use a fully automatic spotter to spot Cy3 and Cy5...
Embodiment 2
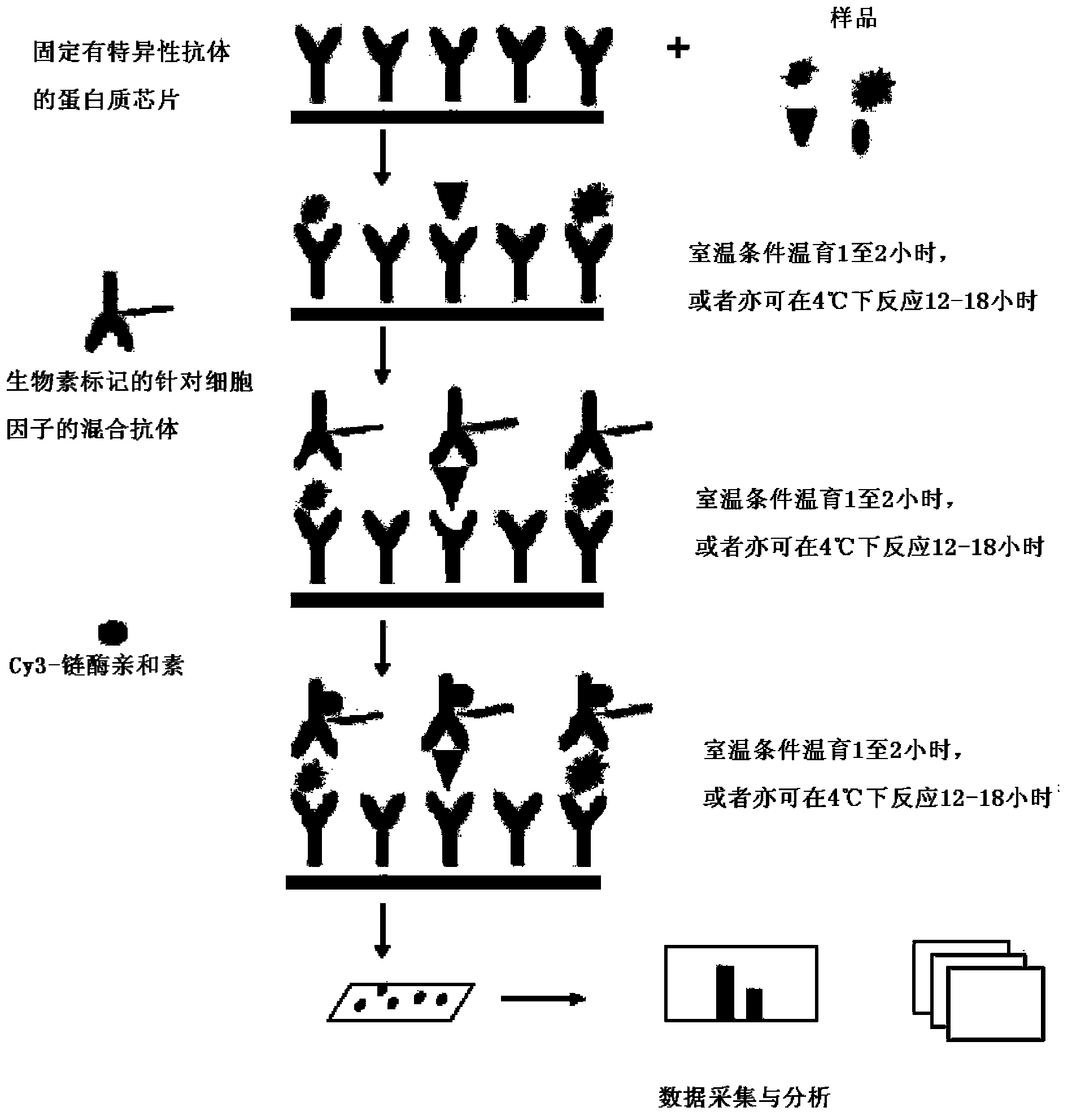
[0034] Example 2: Preparation of an antibody chip kit for simultaneous quantitative detection of multiple cytokines.
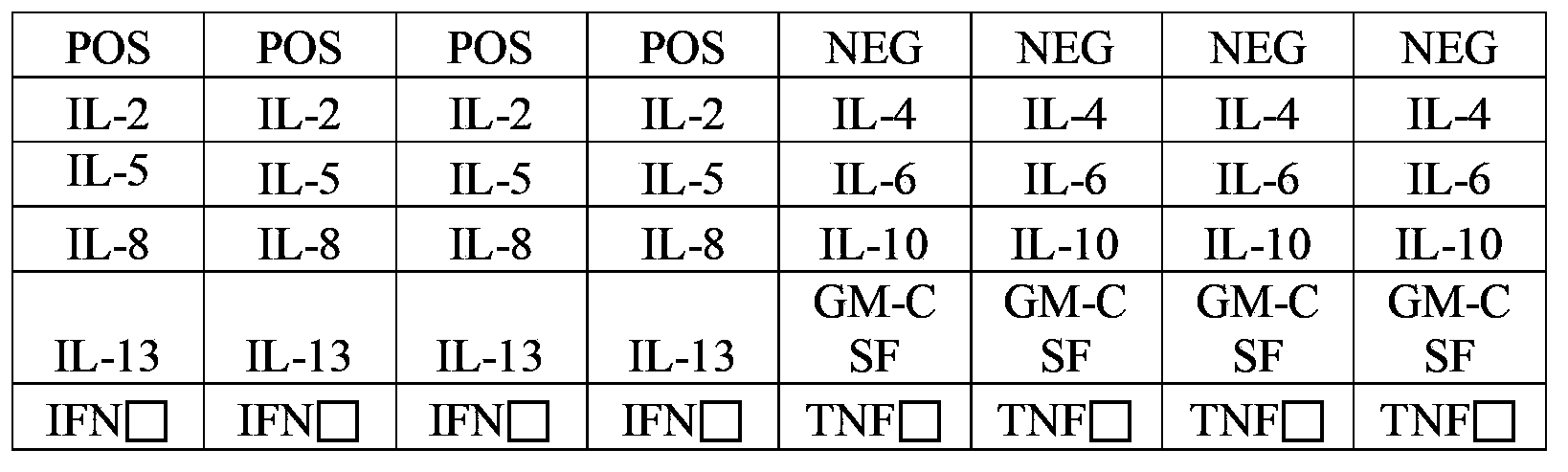
[0035] In order to detect whether there are corresponding cytokines in the sample, prepare slides immobilized with specific antibodies against the following proteins: granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFNgamma), interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 8 (IL-8), interleukin 10 (IL-10), interleukin 13 (IL -13), tumor necrosis factor alpha (TNFalpha).
[0036] 1. Antibody preparation:
[0037] Using specific antibodies against the proteins listed in Table 1, the sources, concentrations, and names of the proteins they target are detailed in Table 1:
[0038] Table 1 The name of the antigenic protein targeted by the specific antibody, the source and concentration information of the antibody
[0039]
[0040] 2. Preparation and storage of antibody chips
[0...
Embodiment 3
[0049] Embodiment 3: Experiment of quantitatively detecting cytokines with the kit of the present invention.
[0050] 1. Complete drying of the slide chip
[0051] Take the slide chip out of the box, and after equilibrating at room temperature for 20-30 minutes, open the packaging bag, peel off the sealing strip, and then place the chip in a vacuum desiccator or dry at room temperature for 1-2 hours.
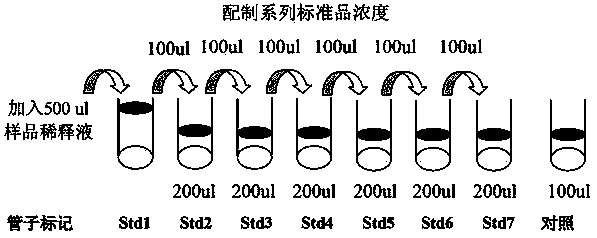
[0052] 2. Press image 3 Perform serial dilutions of cytokine standard gradients as shown in
[0053] 2.1. Add 500 μl of sample diluent to the vial of the cytokine standard mixture, and redissolve the standard. Before opening the vial, give it a quick centrifuge and gently pipet up and down to dissolve the powder. Label this vial as Std1.
[0054] 2.2. Mark 6 clean centrifuge tubes as Std2, Std3 to Std7 respectively, and add 200 μl of sample diluent to each small tube.
[0055] 2.3. Take 100 μl of Std1 and add it to Std2 and mix gently, then take 100 μl from Std2 and add it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com