Tumor-targeting pH-sensitive polymeric micelle composition
A technology of polymer glue and composition, which is applied in the direction of drug combination, antineoplastic drugs, and medical preparations of non-active ingredients, etc., which can solve the problems of insufficient drug accumulation and achieve the effect of effectiveness and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
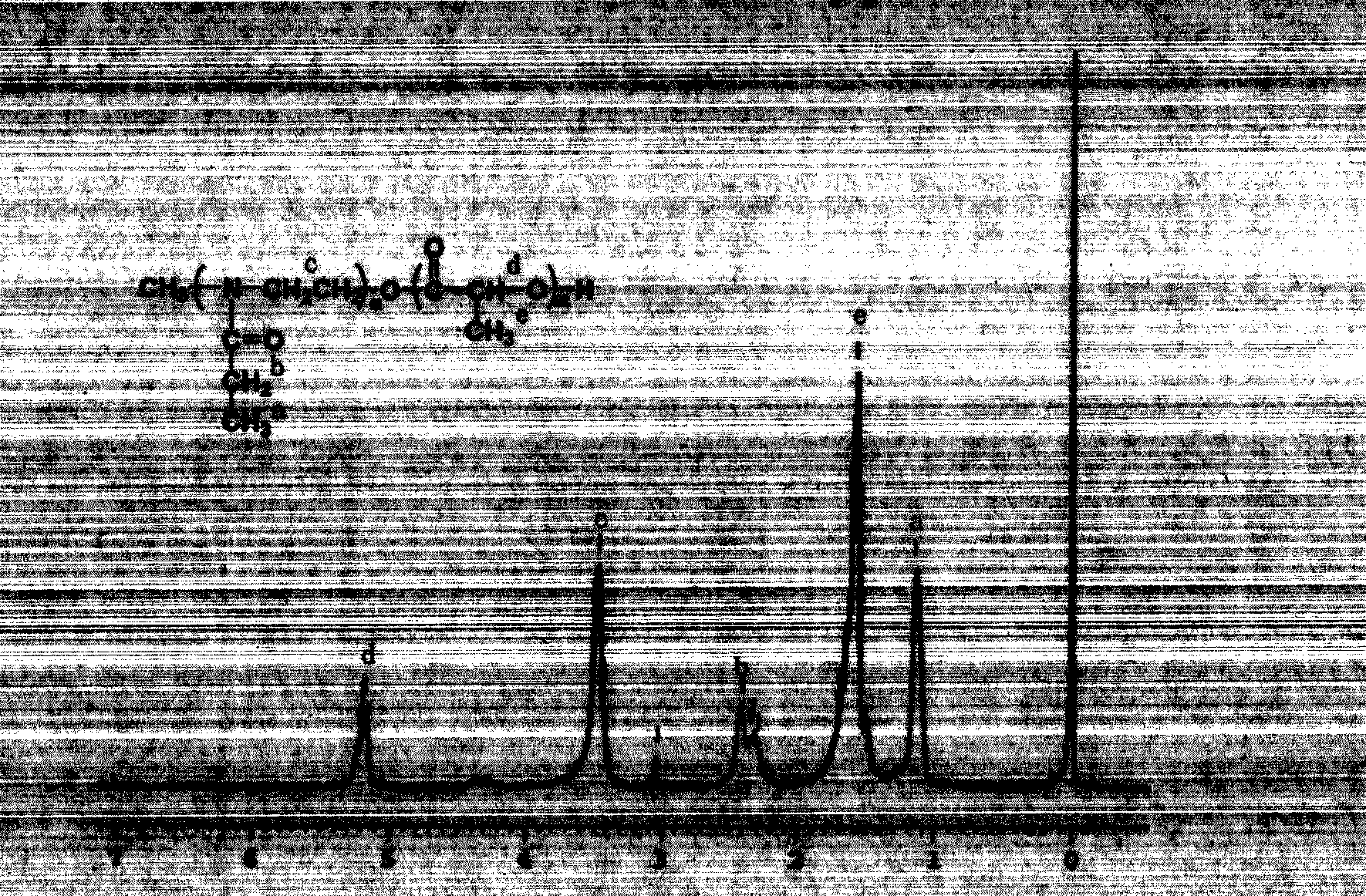
[0040] Example 1 Synthesis of poly(2-ethyl-2-oxazoline)-polylactic acid (PEOz4300-PLA2700)
[0041] In a three-necked flask equipped with a stirrer, 2-ethyl-2-oxazoline (10g, 150mmol), acetonitrile (40mL), methyl p-toluenesulfonate (0.35g) were added, and at an oil bath temperature of 80°C Under a nitrogen atmosphere, the reaction was stirred for 30 h. After cooling, after adding KOH in methanol solution, the reaction was continued for 4h. The solvent was removed, the residue was dissolved in THF, passed through a silica gel column, the effluent was poured into excess cold ether for precipitation, suction filtered, and vacuum-dried for 12 hours. The lactide was recrystallized three times with ethyl acetate, dried under vacuum at room temperature, and its melting point was determined to be 127°C. Put the obtained PEOz-OH powder into a 100mL round-bottomed flask, add about 70mL of toluene, use a water separator to azeotropically remove water, and stop heating after the volatil...
Embodiment 2
[0042] Example 2 Synthesis of poly(2-ethyl-2-oxazoline)-polylactic acid-poly(2-ethyl-2-oxazoline) (PEOz5000-PLA3000-PEOz5000)
[0043] In a three-necked flask equipped with a stirrer, 2-ethyl-2-oxazoline (10g, 150mmol), acetonitrile (40mL), methyl p-toluenesulfonate (0.47g) were added, and at an oil bath temperature of 80°C Under a nitrogen atmosphere, the reaction was stirred for 30 h. After cooling, after adding KOH in methanol solution, the reaction was continued for 4h. The solvent was removed, the residue was dissolved in THF, passed through a silica gel column, the effluent was poured into excess cold ether for precipitation, suction filtered, and vacuum-dried for 12 hours. The obtained PEOz-OH powder (4g) was dissolved in chlorobenzene (80mL), D, L-lactide (6.3g) and stannous octoate (0.63g) were added under a nitrogen atmosphere, and reacted at 140°C for 24h. The reaction solution was poured into excess diethyl ether for precipitation, filtered, and vacuum-dried at r...
Embodiment 3
[0044] Example 3 Synthesis of polylactic acid-poly(2-ethyl-2-oxazoline)-polylactic acid (PLA2000-PEOz8000-PLA2000)
[0045] 2-Ethyl-2-oxazoline (9.9g) and 1,4-dibromo-2-butene (420mg) were dissolved in acetone (40mL), stirred and refluxed at 100°C for 20h under nitrogen atmosphere. After cooling to room temperature, 0.1 mol / L KOH in methanol solution (40 mL) was added to the reaction flask, and the reaction was continued for 4 h. After passing through a silica gel column, the effluent was poured into excess cold diethyl ether for precipitation, suction filtered, and vacuum-dried for 24 hours. Dissolve the obtained HO-PEOz-OH powder (2g) in chlorobenzene (20mL), add D,L-lactide (0.58g) and stannous octoate (30mg) under a nitrogen atmosphere, and react at 140°C for 24h . The reaction solution was poured into excess diethyl ether to precipitate, filtered, and vacuum-dried at room temperature for 12 hours to obtain the triblock copolymer PLA2000-PEOz8000-PLA2000 of the present i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com