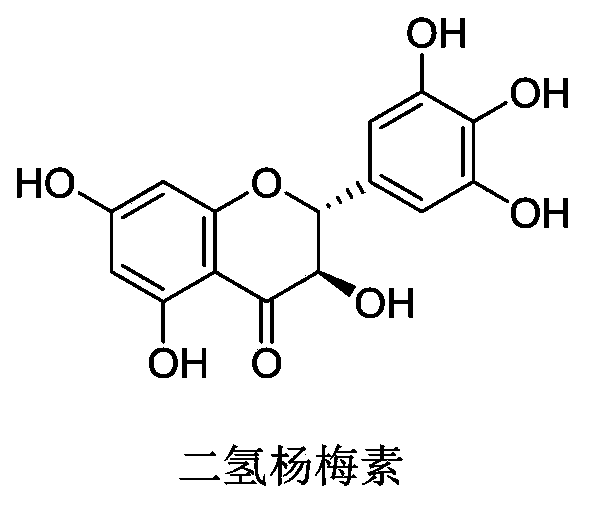
Application of dihydromyricetin to prepare medicines treating Parkinson's syndrome as active composition
A technology for dihydromyricetin and Parkinson's disease, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
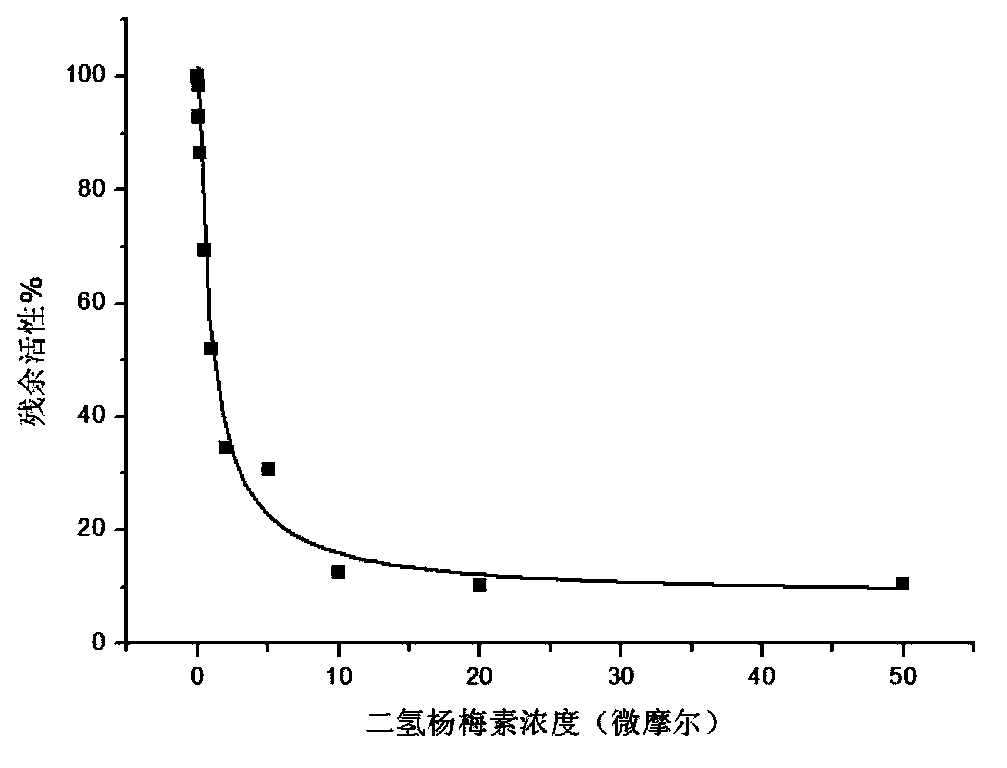
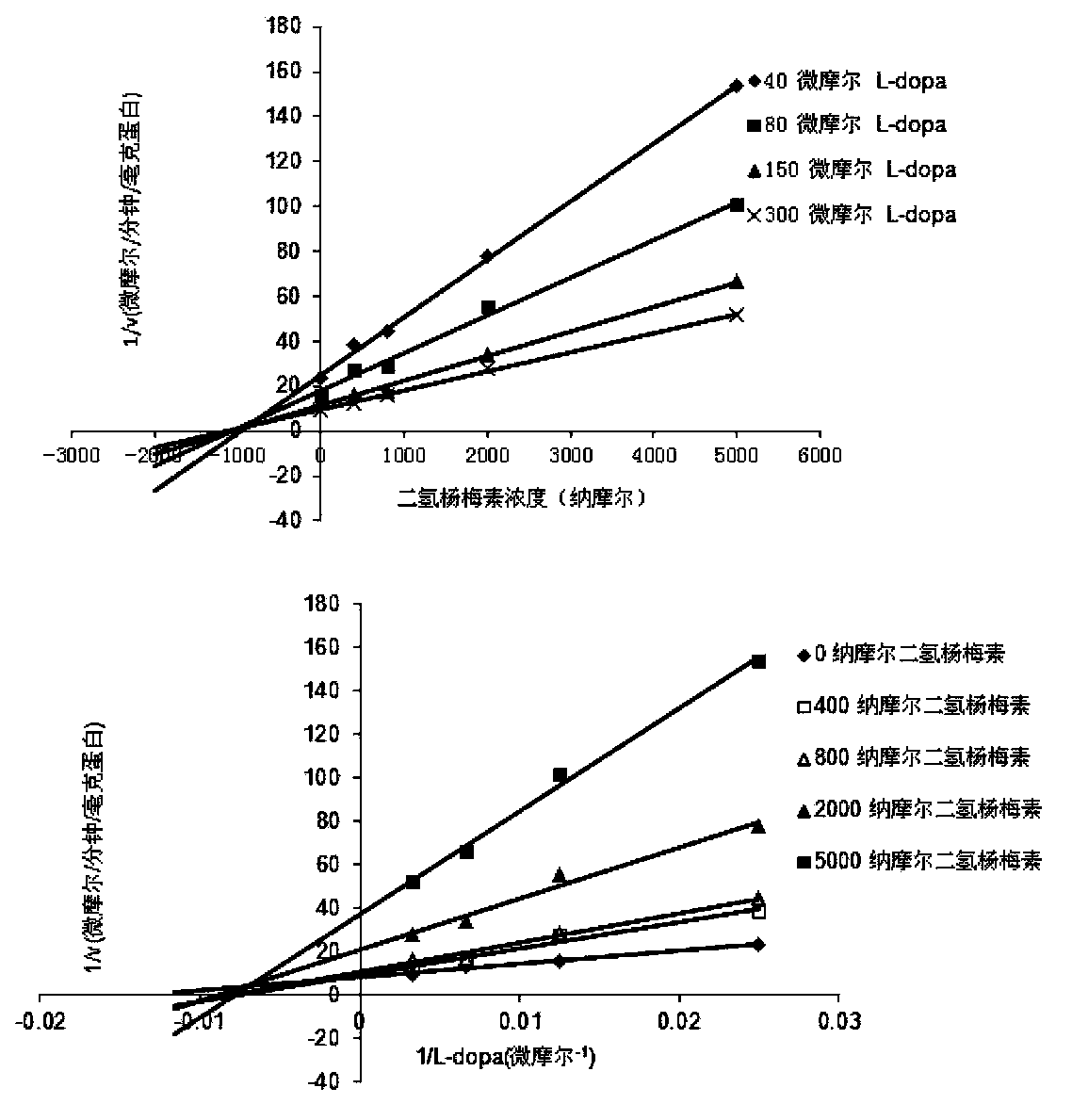
[0015] Using the L-dopa methylation reaction as a probe reaction, with the help of human liver cell plasma in vitro incubation system, dihydromyricetin and plant extracts containing dihydromyricetin (total flavonoids of vine tea, containing 48% dihydromyricetin ) IC for COMT enzyme inhibition 50 , the specific experimental procedure is as follows:
[0016] (1) Add 5mM MgCl to 200 microliters of in vitro metabolic reaction system 2 , 2mM dithiothreitol, 150μM substrate L-dopa, human liver cell plasma protein concentration is 1mg / ml, inhibitor final concentration range is 0.05μM-50μM, pre-incubated at 37℃ for 3 minutes;
[0017] (2) Add S-adenosylmethionine (final concentration: 0.2mM) to the reaction system to initiate the reaction; after reacting at 37°C for 30 minutes, add 200 μl of acetonitrile, shake vigorously, and terminate the reaction;
[0018] (3) Using a high-speed refrigerated centrifuge, under the condition of 20,000×g, centrifuge the above system for 20 minutes a...
Embodiment 2
[0021] Select Kunming mice (purchased from Experimental Animal Center of Dalian Medical University), half male and half male, weighing 18-21 g. The mice were divided into random groups, 20 in each group, half male and half male, and oral acute toxicity tests of dihydromyricetin and total flavonoids of rattan tea were carried out in mice respectively. Dihydromyricetin and vine tea total flavonoids extract were suspended in 0.5% CMC-Na. The experimental groups included different doses of dihydromyricetin (0.1-2g / kg); different doses of total flavonoids of rattan tea (0.1-2g / kg) and 0.5% CMC-Na group. Calculation of LD50 found that the LD50 value of dihydromyricetin and the total flavonoid extract of vine tea containing dihydromyricetin (containing 76% dihydromyricetin) to mice was greater than 2.0g / kg, which belonged to the non-toxic level. In addition, in the human liver microsome incubation system with the addition of the cofactor NADPH or its production system, dihydromyrice...
Embodiment 3
[0023] Select 18 Wistar rats, half male and half female, weighing 180-220g, and randomly divided into 3 groups (oral L-dopa / carbidopa control group, rattan tea total flavonoids / L-dopa / carbidopa group, two Hydromyricetin / L-dopa / carbidopa group), 6 rats / group to carry out the overall pharmacokinetic study of L-dopa. Among them, the total flavonoids of vine tea contain 76% of dihydromyricetin, the dosage is 2g / kg, and the oral dosage of dihydromyricetin is 2g / kg. About 0.5ml of rat plasma samples were collected before administration and at 5, 10, 15, 30, 60, 120, 180, and 240 minutes after administration, and placed in pre-heparinized 1.5ml pointed-bottomed brown centrifuge tubes , after centrifugation at 4000×g for 10 minutes, separate the plasma, add an equal volume of methanol to precipitate protein and centrifuge at a high speed (20,000×g), take the supernatant and store it in a -80°C refrigerator for testing. UFLC-MS / MS was used to measure the blood concentration of L-dopa ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com