Fluoride phosphate matrix luminescent material and preparation method thereof
A technology of luminescent materials and fluorophosphate, which is applied in the direction of luminescent materials, chemical instruments and methods, can solve the problems of limited application in lighting and display fields, low luminous efficiency, etc., and achieve improved luminous efficiency, fewer process steps, and better process conditions. harsh effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
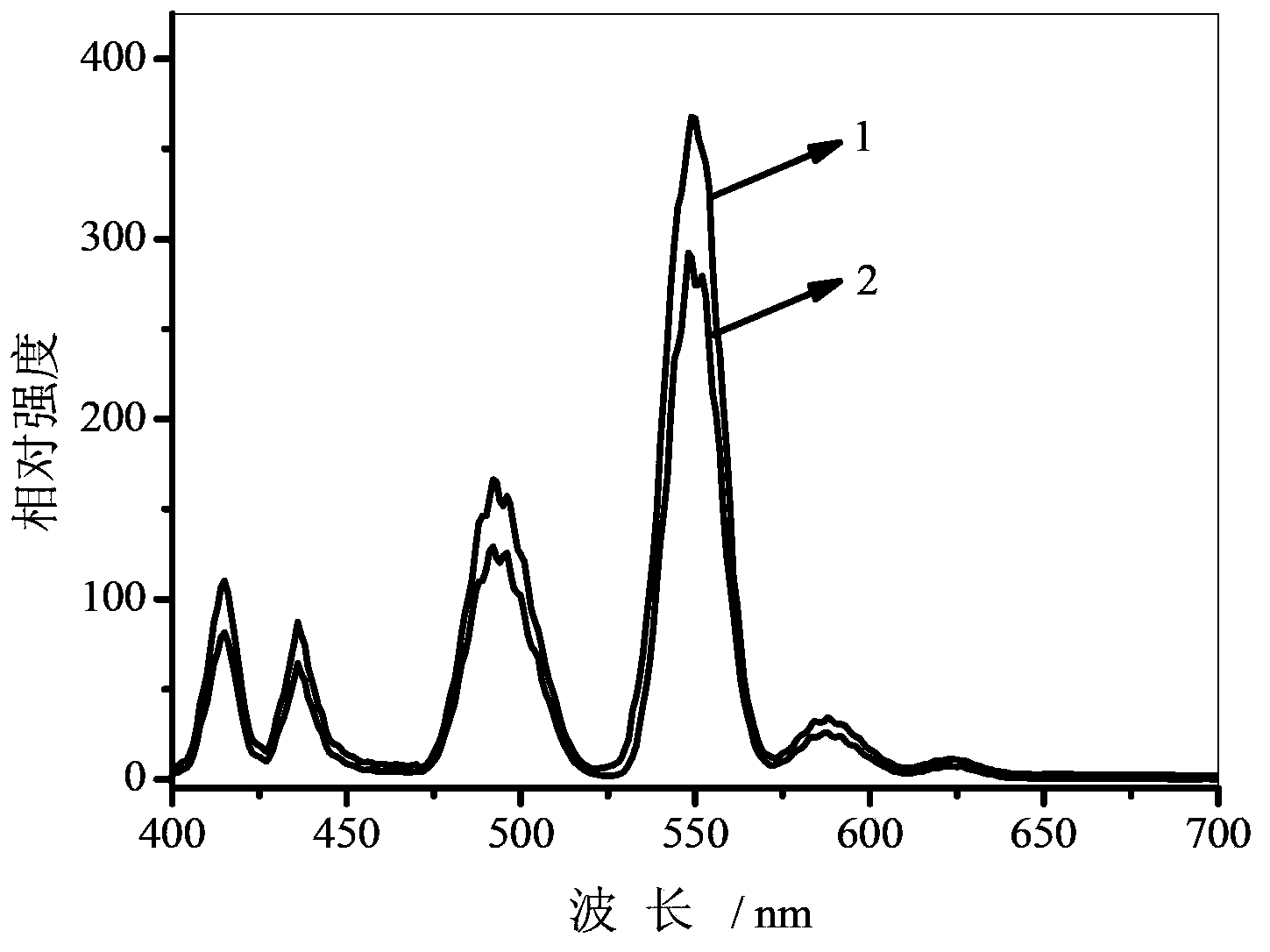
Image
Examples
Embodiment 1
[0026] Preparation of Na by sol-gel method 2 Gd 0.99 PO 4 f 2 :Tb 0.01 3+ ,Pd 1×10ˉ5 :
[0027] Preparation of Pd nanoparticle sol: Weigh 0.22 mg palladium chloride (PdCl 2 2H 2 O) Dissolved in 10mL of deionized water; when the palladium chloride was completely dissolved, weigh 11.0mg of sodium citrate and 4.0mg of sodium lauryl sulfate, and dissolve them in an aqueous solution of palladium chloride under a magnetic stirring environment; Weigh 0.38mg sodium borohydride and dissolve it in 100mL deionized water to obtain a concentration of 1×10 -4mol / L sodium borohydride reducing solution; in the environment of magnetic stirring, quickly add 10mL1×10 -4 sodium borohydride aqueous solution, and then continue to react for 20min to obtain 20mL Pd content of 5×10 -5 mol / L Pd nanoparticle sol.
[0028] Weigh 1.0886g CH 3 COONa·3H 2 O, 1.3239g Gd(CH 3 COO) 3 and 0.0132gTb(CH 3 COO) 3 Put it in a container, then add 50mL of a mixed solution of ethanol and water with a ...
Embodiment 2
[0031] Preparation of Na by sol-gel method 2 Gd 0.6 PO 4 f 2 :Tb 0.4 3+ ,Au 1×10 -2
[0032] Preparation of Au nanoparticle sol: Weigh 41.2 mg of chloroauric acid (AuCl 3 ·HCl·4H 2 O) Dissolve in 10mL of deionized water; when the chloroauric acid is completely dissolved, weigh 14mg of sodium citrate and 6mg of cetyltrimethylammonium bromide, and dissolve them into the chloroauric acid aqueous solution under magnetic stirring Medium; Weigh 3.8mg of sodium borohydride and 17.6mg of ascorbic acid and dissolve them in 10mL deionized water respectively to obtain 10mL concentration of 1×10 -2 mol / L sodium borohydride aqueous solution and 10mL concentration is 1×10 -2 mol / L ascorbic acid aqueous solution; in the environment of magnetic stirring, first add 5mL sodium borohydride aqueous solution to the chloroauric acid aqueous solution, stir and react for 5min, then add 5mL1×10 -2 mol / L ascorbic acid aqueous solution, and then continue to react for 30min to obtain 20mL Au co...
Embodiment 3
[0036] Preparation of Na by sol-gel method 2 Gd 0.9 PO 4 f 2 :Tb 0.1 3+ , Ag 2.5×10 -4:
[0037] Preparation of Ag nanoparticles sol: weigh 3.4 mg silver nitrate (AgNO 3 ) into 18.4mL of deionized water; when the silver nitrate is completely dissolved, weigh 42mg of sodium citrate and dissolve it in the silver nitrate aqueous solution under magnetic stirring; weigh 5.7mg of sodium borohydride and dissolve it in 10mL of deionized water, Obtain 10mL concentration as 1.5×10 -2 mol / L sodium borohydride aqueous solution; under the environment of magnetic stirring, add 1.6mL1.5×10 -2 mol / L sodium borohydride aqueous solution, and then continue to react for 10min to obtain 20mL Ag content of 1×10 -3 mol / L of Ag nanoparticles sol.
[0038] Weigh 0.6799g NaNO 3 , 1.2356g Gd(NO 3 ) 3 and 0.1380g Tb(NO 3 ) 3 Put it in a container, then add 50mL of a mixed solution of ethanol and water with a volume ratio of 8:1, and add 1mL with a concentration of 1×10 under the condition ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com