Duck plague virus recombinant vaccine strain expressing enhanced green fluorescent protein gene, constructing method thereof and applications of the recombinant vaccine strain
A technology of green fluorescent protein and duck plague virus, applied in the field of biomedicine, can solve the problem that the construction of recombinant virus of foreign genes has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The construction of embodiment 1 transfer vector
[0035] According to the flanking sequence of the US10 gene sequence of the DEV virus genome (GenBank accession number is EF524095), 2 pairs of primers were designed using Oligo6.0 software. The primer sequences are as follows:
[0036] VL1 (upstream): 5'-ATCGATTGACGATGAGCGATCGGAAT-3'
[0037] VL2 (downstream): 5'-CCTAGGGTTGCGCGTTGTGTATAAGT-3'
[0038] VR1 (upstream): 5'-ACGCGTGACTCTGACTGATACTCTAC-3'
[0039] VR2 (downstream): 5'-ATGCATCTAATCGGTTATTTGCTGCT-3'
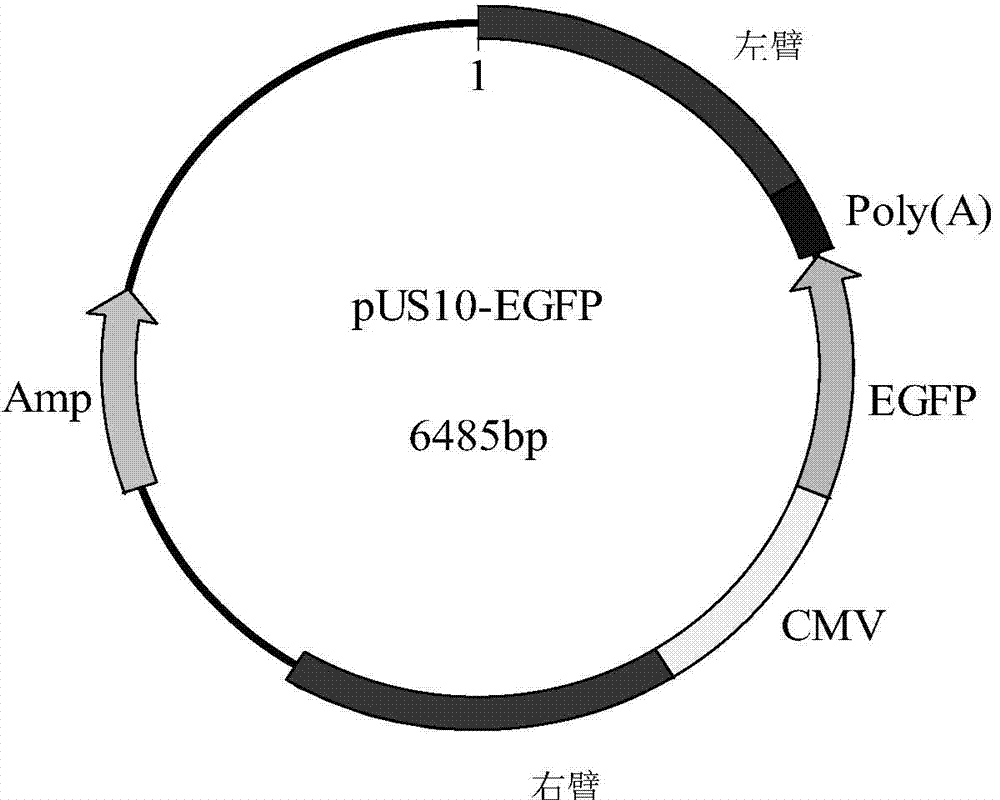
[0040] First, primers VL1 (upstream) and VL2 (downstream) were used to amplify the left homology arm, primers VR1 (upstream) and VR2 (downstream) were used to amplify the right homology arm, and the left homology arm (Cla I+Bln I) and The right homology arms (Mlu I+Ava III) were respectively inserted into the pT-EGFP vector of the EGFP expression cassette (CMV-EGFP) with the CMV promoter constructed by our laboratory to construct the transfer vector pUS10-EGFP ...
Embodiment 2
[0041] The extraction of embodiment 2 duck plague virus genome
[0042] Inoculate DEV Clone-03 cytotoxicity at an MOI of 0.001 in a 5mL cell flask covered with a CEF monolayer (the preparation of chicken embryo fibroblasts refers to the operation of "Principles and Techniques of In Vitro Culture" (Xue Qingshan, 2001)), 37°C Adsorb for 2 hours, discard the virus solution, and replace the DMEM cell maintenance solution (containing 2% FBS), and when the cytopathy reaches 80% to 90%, discard the cell maintenance solution, and add the cell digestion solution (1860 μL STE; 100 μL 10% SDS; 40 μL Proteinase K20mg / mL), digest overnight at 37°C, add an equal volume of phenol for extraction once, an equal volume of phenol chloroform (1:1) for extraction once, add an equal volume of chloroform for extraction once; add 1 / 10 volume of NaAC (3M, pH5 .2), 2.5 times the volume of absolute ethanol, placed at -20°C for precipitation overnight, centrifuged at 4°C for 15 minutes, after air-drying,...
Embodiment 3
[0043] Example 3 Transfection
[0044] Day1: Prepare cells
[0045] The CEF cells were subcultured in advance and spread in 5mL cell flasks, cultured in a 2% constant temperature incubator at 37°C.
[0046] Day2: Transfection
[0047] (1) Change the cell culture medium 3-4 hours before transfection.
[0048] (2) Transfection system: Solution A: 18 μL 2M CaCl2, 10 μg DNA (the ratio of transfer vector to viral genome is 3:1), add deionized water to make up the volume to 150 μL. Solution B: 150 μL 2×Hepes Buffered Saline (HBS).
[0049] (3) Use a pipette to add liquid A to liquid B drop by drop, while adding liquid A, use another pipette to slowly blow air into liquid B. This process should be completed within 1-2 minutes.
[0050] (4) Incubate the A and B mixture at room temperature for 30 min.
[0051] (5) Add the mixed solution into the cell culture medium.
[0052] (6) Place the cells in a 2% constant temperature incubator at 37°C for further culture.
[0053] Day3: Ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com