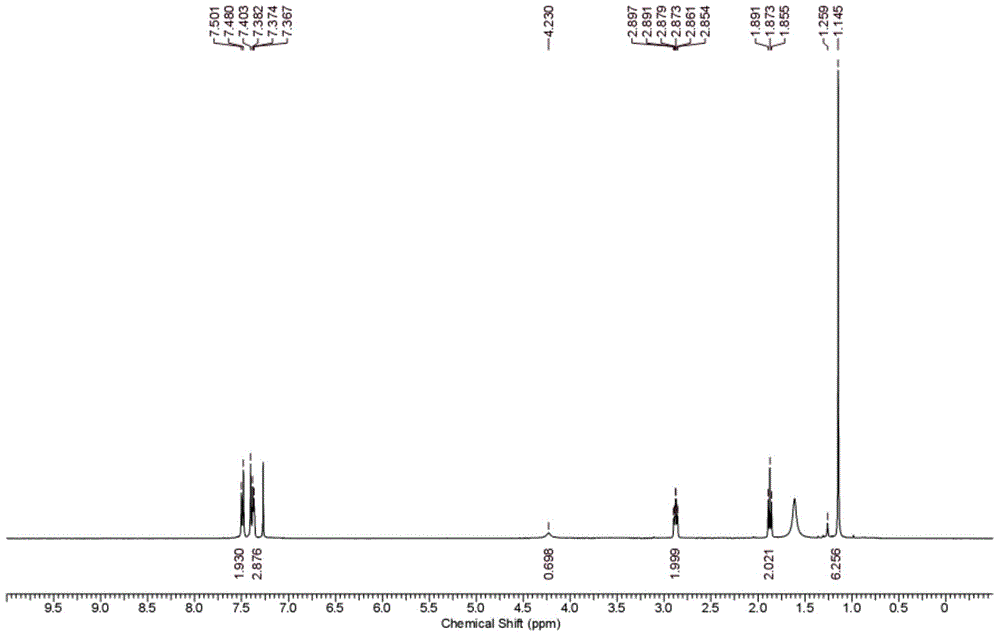
Preparation method for triticonazole intermediate
An intermediate, the technology of Fenbenazole, applied in the direction of condensation preparation of carbonyl compounds, organic chemistry, etc., can solve problems such as difficult production, and achieve the effects of improving purity, controlling impurity content, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
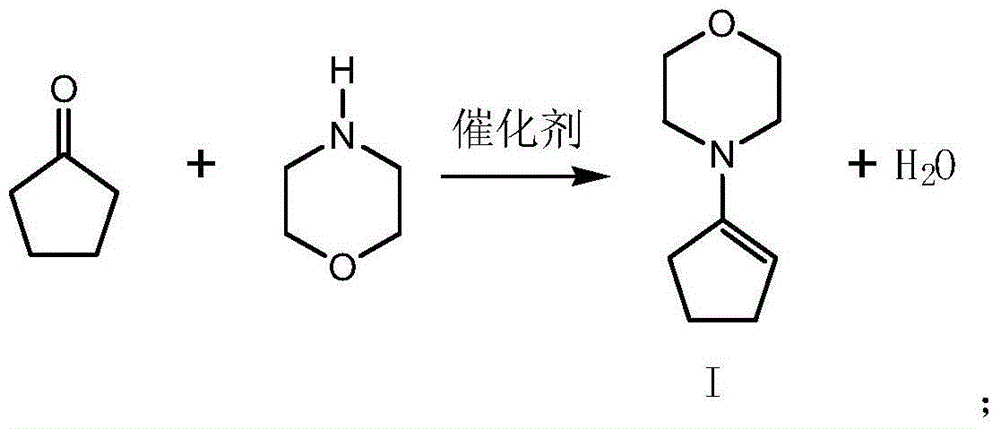
[0039] This example provides a kind of preparation method of 5-(4-chlorobenzylidene)-2,2-dimethylcyclopentanone, which comprises the steps:
[0040] (1) Oximation reaction
[0041] Add 10.9 g of cyclopentanone, 12.8 g of morpholine, 0.2 g of p-toluenesulfonic acid, and 50 ml of toluene into a 250 ml dry three-necked flask, and react at 70° C. for 5 hours. Follow the reaction by TLC until the cyclopentanone is completely reacted. Wash with water, separate layers, and desolventize the toluene layer to 100°C to obtain 19.5 g of residue, which is Intermediate I. Purity (HPLC) 98.9%, yield: 98.2% (calculated as cyclopentanone).
[0042] (2) Condensation reaction
[0043] Add 19.5 grams of intermediate I and 18 grams of p-chlorobenzaldehyde in a 250 ml dry three-necked flask, 65 ml of toluene, and react at 60°C for 2 hours, follow the reaction by TLC until the intermediate I completely disappears, distill the toluene under reduced pressure to an internal temperature of 80 °C, 33 ...
Embodiment 2
[0049] This example provides a kind of preparation method of 5-(4-chlorobenzylidene)-2,2-dimethylcyclopentanone, which comprises the steps:
[0050] (1) Oximation reaction
[0051] Add 10.9 g of cyclopentanone, 12.8 g of morpholine, 0.2 g of anhydrous aluminum sulfate, and 50 ml of cyclohexane into a 250 ml dry three-necked flask, and react under reflux for 5 hours, and follow the reaction by TLC until the cyclopentanone is completely reacted. Washed with water, separated into layers, and the cyclohexane layer was precipitated under negative pressure to an internal temperature of 80° C. to obtain 18.8 g of a residue, which is Intermediate I. HPLC purity: 97.3%, yield: 92.2% (calculated as cyclopentanone).
[0052] (2) Condensation reaction
[0053] Add 18.8 g of intermediate I, 17.4 g of p-chlorobenzaldehyde, and 95 g of methylcyclohexane into a 250 ml dry three-necked flask, react at 50° C. for 2 hours, follow the reaction by TLC until intermediate I completely disappears. ...
Embodiment 3
[0059] Add 10.9 g of cyclopentanone, 12.8 g of morpholine, 0.2 g of anhydrous aluminum sulfate, and 50 ml of cyclohexane into a 250 ml dry three-necked flask, and react at 40° C. for 5 hours. Follow the reaction by TLC until the cyclopentanone is completely reacted. Washed with water, separated into layers, and the cyclohexane layer was precipitated under negative pressure to an internal temperature of 80° C. to obtain 18.9 g of a residue, which is Intermediate I. HPLC purity: 96%, yield: 91.4% (calculated as cyclopentanone).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com