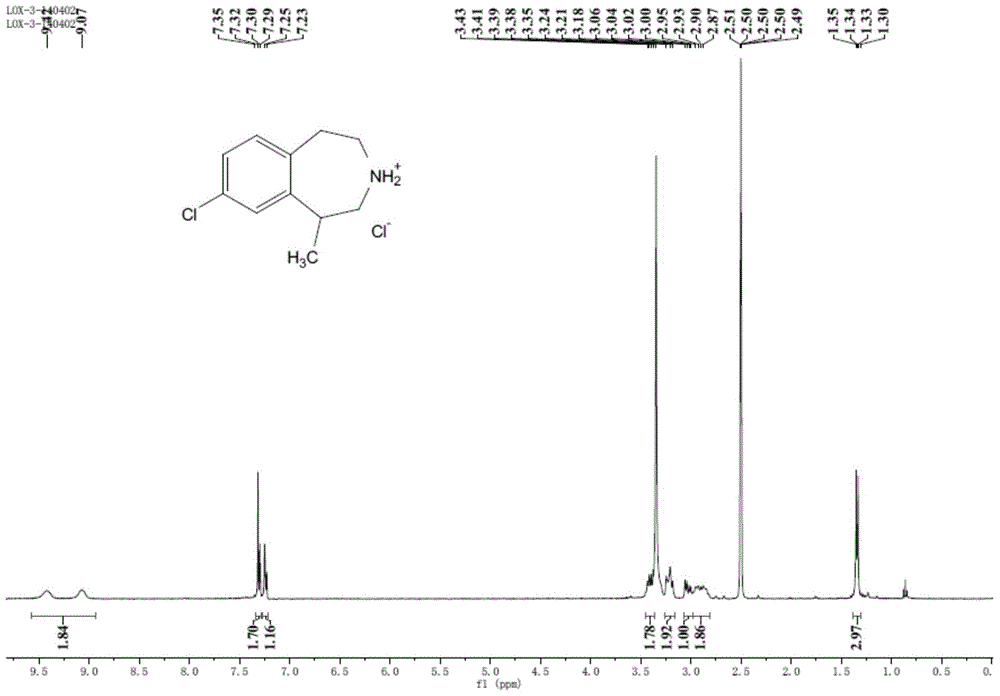
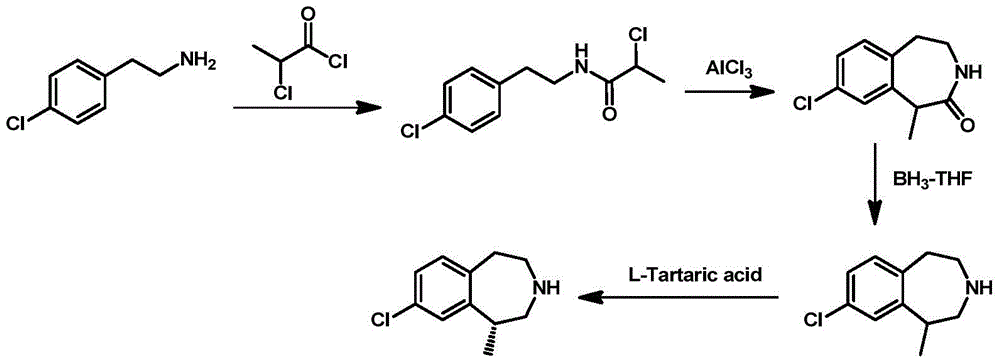
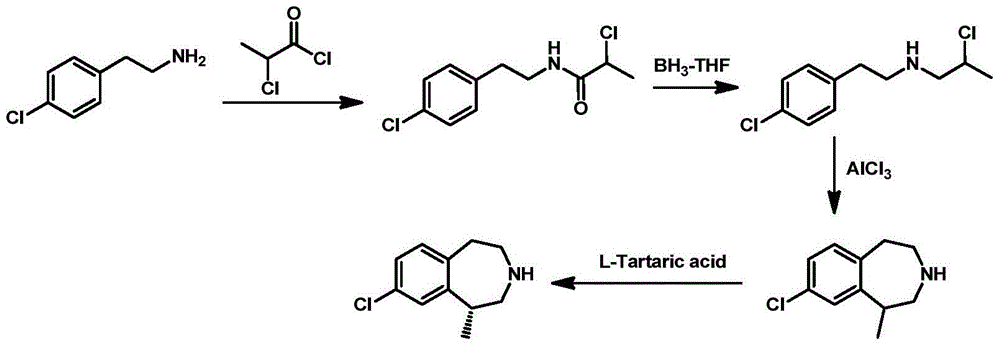
Preparation method of 8-chloro-1-methyl-2,3,4,5-tetrahydro-1h-3-benzazepine
A technology of chlorophenylacetonitrile and trimethylchlorosilane, which is applied in the preparation of amino compounds, organic compounds, carboxylic acid amides, etc., and can solve the problems of high cost and difficult to obtain in large quantities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1, the preparation of formula I compound:
[0067] 1.5g of the compound of formula III (6.09mmol) and 1.63g of aluminum chloride (12.19mmol) were placed in a 500mL three-necked flask, heated to 150°C, melted and stirred for 8 hours, then stopped the reaction. The reaction system to prepare the compound of formula II was cooled to room temperature, and 60 mL of treated anhydrous tetrahydrofuran was added under an ice bath to dissolve part of the reactants. After stirring for five minutes, 0.21 g of sodium borohydride (5.72 mmol) was added under an ice bath, and stirred for five minutes. , heated to 60°C and stirred for 48 hours.
[0068] After the reaction is over, under ice bath conditions, slowly add ice water dropwise to quench the reaction, add 10mL of 5% dilute hydrochloric acid and stir for half an hour, add 80mL of ethyl acetate to dilute the system, oscillate and stand for stratification, separate the water phase, add saturated carbonic acid Adjust th...
Embodiment 2
[0069] Embodiment 2, the preparation of formula I compound:
[0070] 1.5g of compound of formula III (6.09mmol) and 1.46g of aluminum chloride (10.97mmol) were placed in a 500mL three-necked flask, heated to 150°C, melted and stirred for 8 hours to stop the reaction. The reaction system to prepare the compound of formula II was cooled to room temperature, and 60 mL of treated anhydrous tetrahydrofuran was added under ice bath to dissolve part of the reactants. After stirring for five minutes, 0.58 g of sodium borohydride (15.26 mmol) was added under ice bath, and stirred for five minutes. , heated to 60°C and stirred for 36 hours.
[0071] After the reaction is over, under ice bath conditions, slowly add ice water dropwise to quench the reaction, add 10mL of 5% dilute hydrochloric acid and stir for half an hour, add 80mL of ethyl acetate to dilute the system, oscillate and stand for stratification, separate the water phase, add saturated carbonic acid Adjust the pH to 9-10 wi...
Embodiment 3
[0072] Embodiment 3, the preparation of formula I compound:
[0073] Place 2.00g of compound of formula III (8.13mmol) and 1.63g of aluminum chloride (12.19mmol) in a 500mL three-necked flask, heat to 150°C, melt and stir for 8 hours, then stop the reaction. The reaction system to prepare the compound of formula II was cooled to room temperature, and 40 mL of 1,2-dichloroethane was added under ice bath to dissolve part of the reactants. After stirring for five minutes, 0.79 g of sodium borohydride (20.99 mmol) was added under ice bath, and stirred for five minutes. minutes, heated to 60°C and stirred for 20 hours.
[0074] After the reaction, under ice bath conditions, slowly add ice water dropwise to quench the reaction, add 10mL of 5% dilute hydrochloric acid and stir for half an hour, add 80mL of ethyl acetate to dilute the system, oscillate and stand for stratification, separate the water phase, add saturated carbonic acid Adjust the pH to 9-10 with sodium solution, extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com