Ophthalmic composition
A composition and ophthalmic technology, applied in the directions of drug combination, drug delivery, medical preparations of inactive ingredients, etc., can solve problems such as the reduction of the content rate, and achieve inhibition of adsorption, inhibition of the reduction of the content rate or concentration, and good stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077] Methods for the preparation of ophthalmic formulations are well known. It can be prepared by mixing GGA with a pharmaceutically acceptable base or carrier, pharmaceutically acceptable additives for ophthalmic preparations, and other active ingredients (physiologically active ingredients or pharmacologically active ingredients other than GGA).
[0078]
[0079] Examples of the base or carrier include water; aqueous solvents such as polar solvents; polyhydric alcohols; vegetable oils; Examples of bases or carriers for intraocular injections include distilled water for injection or physiological saline.
[0080] A base or a carrier may be used alone or in combination of two or more.
[0081]
[0082] Examples of additives include surfactants, fragrances or refreshers, preservatives, bactericides or antibacterial agents, pH adjusters, isotonic agents, chelating agents, buffers, stabilizers, other antioxidants, thickeners, and the like. Intraocular injections may c...
Embodiment
[0157] Hereinafter, although an Example is given and this invention is demonstrated in more detail, this invention is not limited to these Examples.
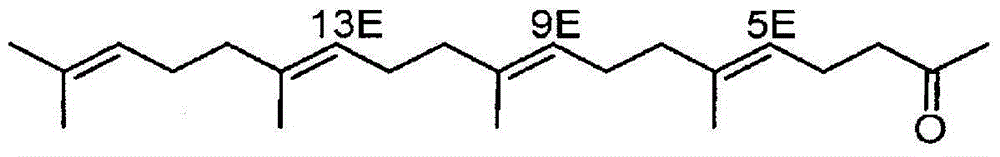
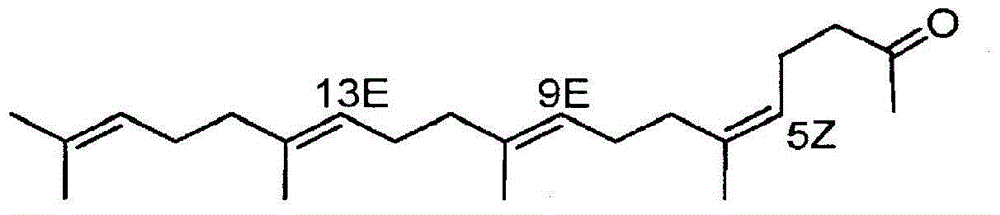
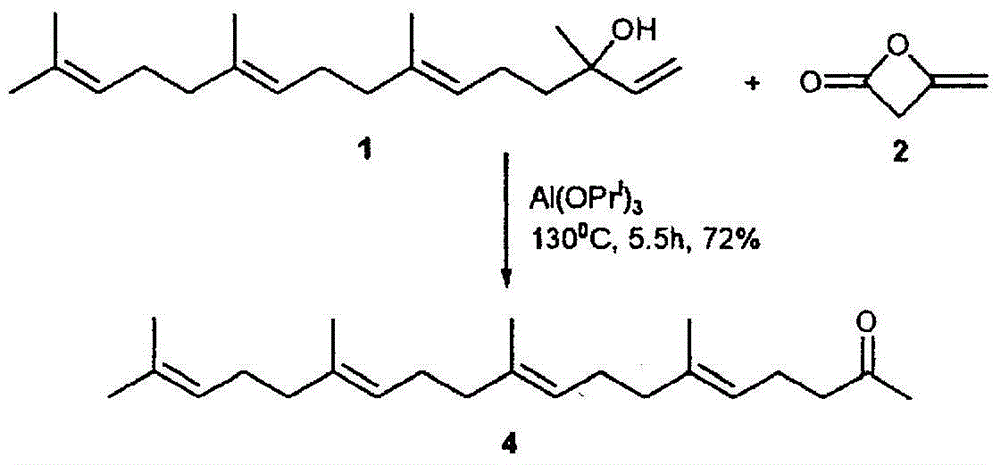
[0158] (1) Preparation of geranylgeranyl acetone
[0159] Commercially available teprenone (Wako Pure Chemical Industries, Ltd.) (all-trans form: 5Z monocis form (weight ratio) = 6:4) was purchased, and the all-trans form was purified by silica gel column chromatography.
[0160] As specific conditions, silica gel (PSQ60B, manufactured by Fuji Silicon Chemical Co., Ltd.) was filled in a glass tube, and fractional purification was performed with a mobile phase (n-hexane:ethyl acetate=9:1). After fractionation, each fraction was concentrated and dried under reduced pressure, and then the purity and structure of the all-trans isomer were confirmed by GC and 1H-NMR (solvent: deuterated chloroform, internal standard: tetramethylsilane) (yield is about 20%).
[0161]
[0162] Chromatographic column: DB-1 (J&Wscientific, 0.53mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com