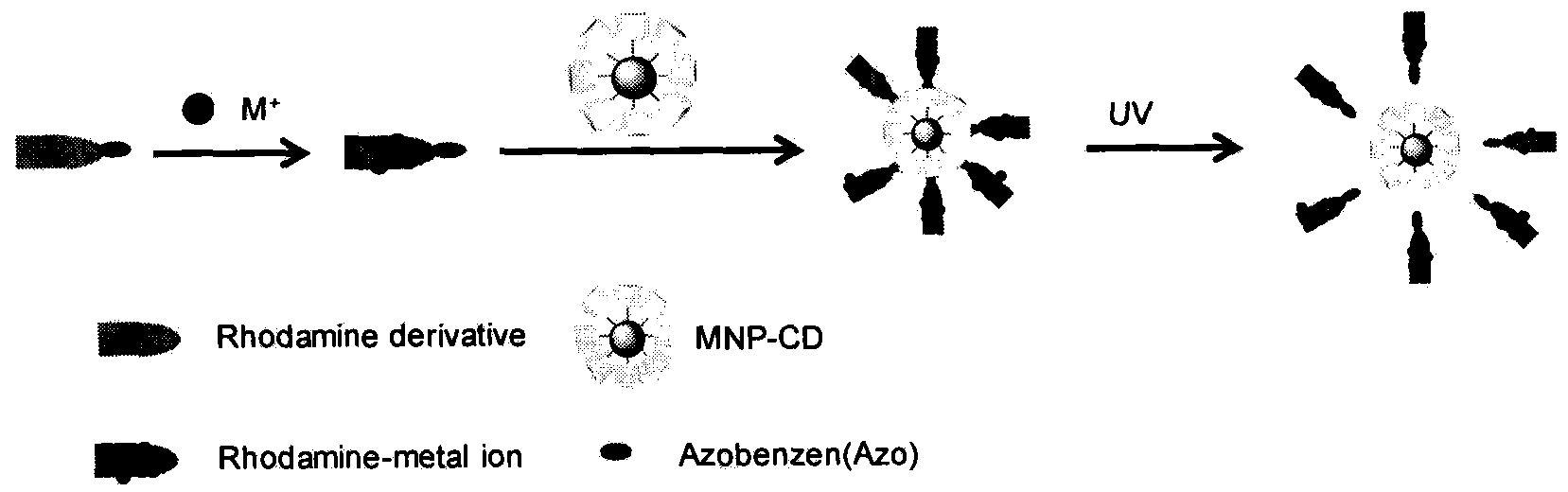
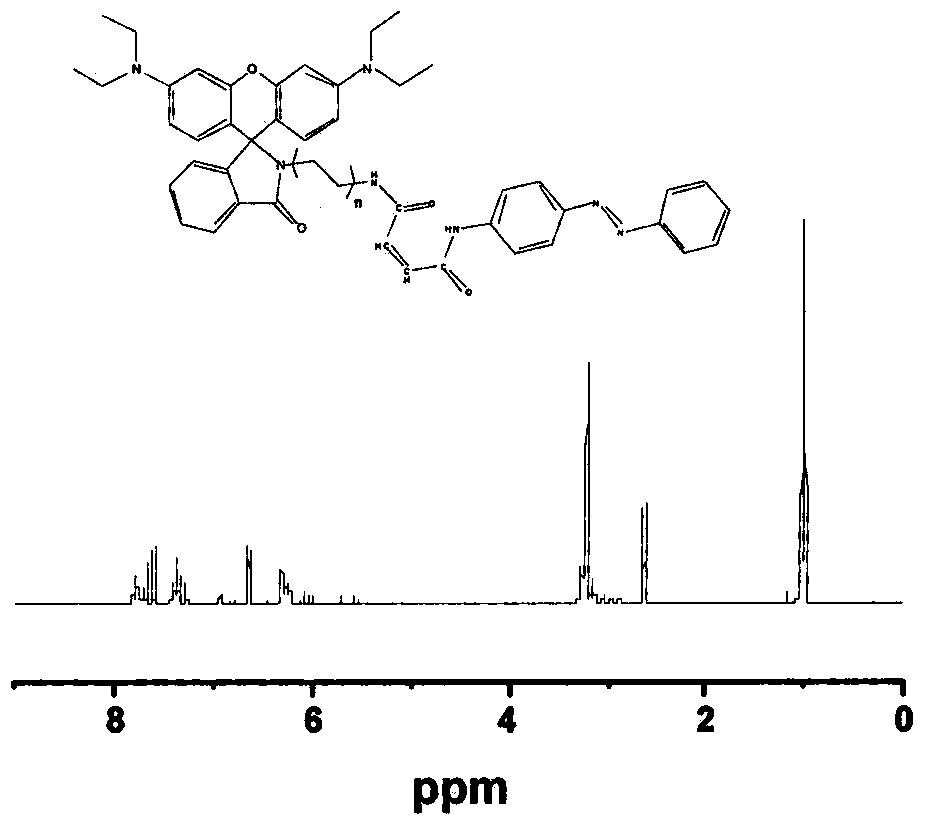
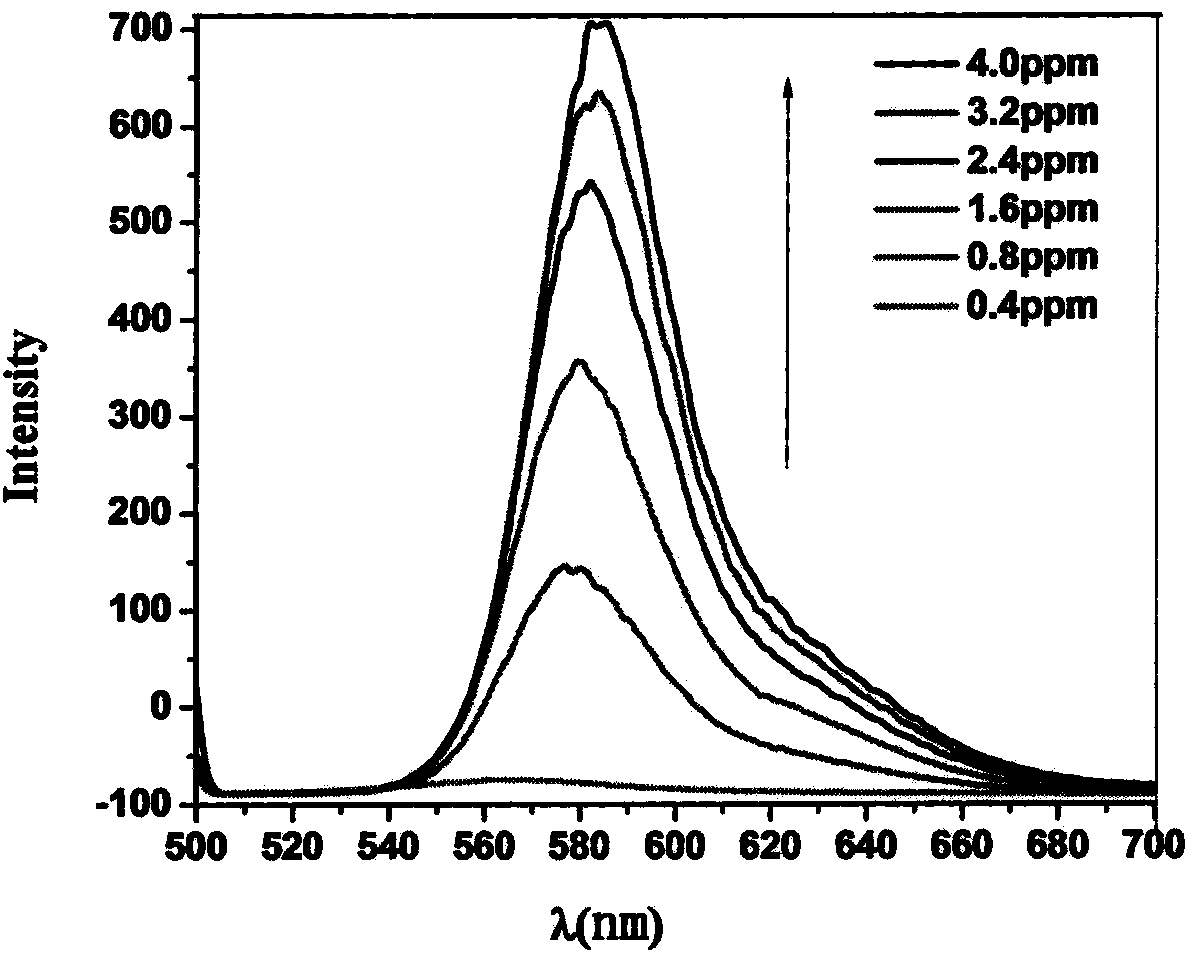
Rhodamine fluorescent probe capable of detecting and separating heavy metal ions, and preparation method of rhodamine fluorescent probe
A technology of heavy metal ions and fluorescent probes, used in fluorescence/phosphorescence, alkali metal compounds, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, the synthesis of rhodamine lactam (SRhB)
[0024] RhB (10.46mmol) was dissolved in 180ml absolute ethanol, N 2 Quickly add 40.23mL of ethylenediamine under the atmosphere, slowly add the temperature to 85°C, react for 24h, and distill under reduced pressure, the obtained powder is distilled with CH 2 Cl 2 (100mL) was dissolved, extracted with water (200mL), the organic layer was separated, washed with water 5 times, and distilled under reduced pressure to obtain an orange powder. Chromatography on silica gel (CH 2 Cl 2 / EtOH / Et 3 N=5:1:0.1) purification, yield 90%.
Embodiment 2
[0025] Embodiment 2, the preparation of azobenzene-rhodamine (SRhB-Azo)
[0026]Add 100mg maleic anhydride (MAH), 516mg SRhB, 20mg DMAP, 5mL DMSO to a 50mL one-necked flask, react at room temperature for 12 hours, add 191mg EDC, 137mg HOBt, activate for 1 hour, add 197.24mg azobenzene (Azo) and react at room temperature for 12 hours . The resulting powder was washed with CH 2 Cl 2 (100mL) was dissolved, extracted with water (200mL), separated the organic layer, and distilled under reduced pressure to obtain a pink powder, which was purified by silica gel chromatography (CH 2 Cl 2 ).
Embodiment 3
[0027] Embodiment 3, detect mercury ion
[0028] Configure 2.5×10 in a 100mL volumetric flask -3 M's SRhB-MAH-A 2 O ethanol standard solution, 1×10 -4 M mercury ion standard solution. Take 1mL of SRhB-Azo standard solution and 0.1mL, 0.2mL, 0.4mL, 0.6mL, 0.8mL, 1.0mL of mercury ion standard solution in a centrifuge tube, dilute to 5mL with ethanol, measure the fluorescence intensity at 492nm, and carry out at 563nm Photometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com