Preparation of interface self assembled carbonyl reductase and application thereof to S-eslicarbazepine synthesis
A carbonyl reductase, self-assembly technology, applied in oxidoreductase, microorganism-based methods, enzymes, etc., can solve the problems of low substrate conversion efficiency, low catalytic water solubility, low water solubility of organic substrates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the preparation of carbonyl reductase
[0020] (1) Disperse Bacillus anthracis CGMCC No.12337 in 50ml Tris-HCl (0.02M, pH6.5) buffer solution, the cell concentration is 2g / L (bacteria dry weight / buffer volume), put in ice water bath (0-4°C) for cell disruption using ultrasound. Place the ultrasonic probe at 1-1.5 cm below the surface of the bacterial suspension, with a power of 400 W, ultrasonic for 2 s with an interval of 4 s, and ultrasonic for 20 min. The crushed sample was centrifuged in a centrifuge at 8000r / min for 10 minutes to obtain the supernatant, which was the crude enzyme solution I.
[0021] (2) The crude enzyme liquid I was subjected to ammonium sulfate fractional precipitation. Pre-cool the crude enzyme solution Ⅰ in an ice-water bath to 0-4°C, and slowly add ammonium sulfate that has been ground into fine powder while stirring gently until the mass saturation of ammonium sulfate is 40%. After the ammonium sulfate is completely dissolved,...
Embodiment 2
[0028] Example 2 Preparation and Application of Interfacial Self-Assembly Carbonyl Reductase
[0029] (1) Preparation of interfacial self-assembled carbonyl reductase
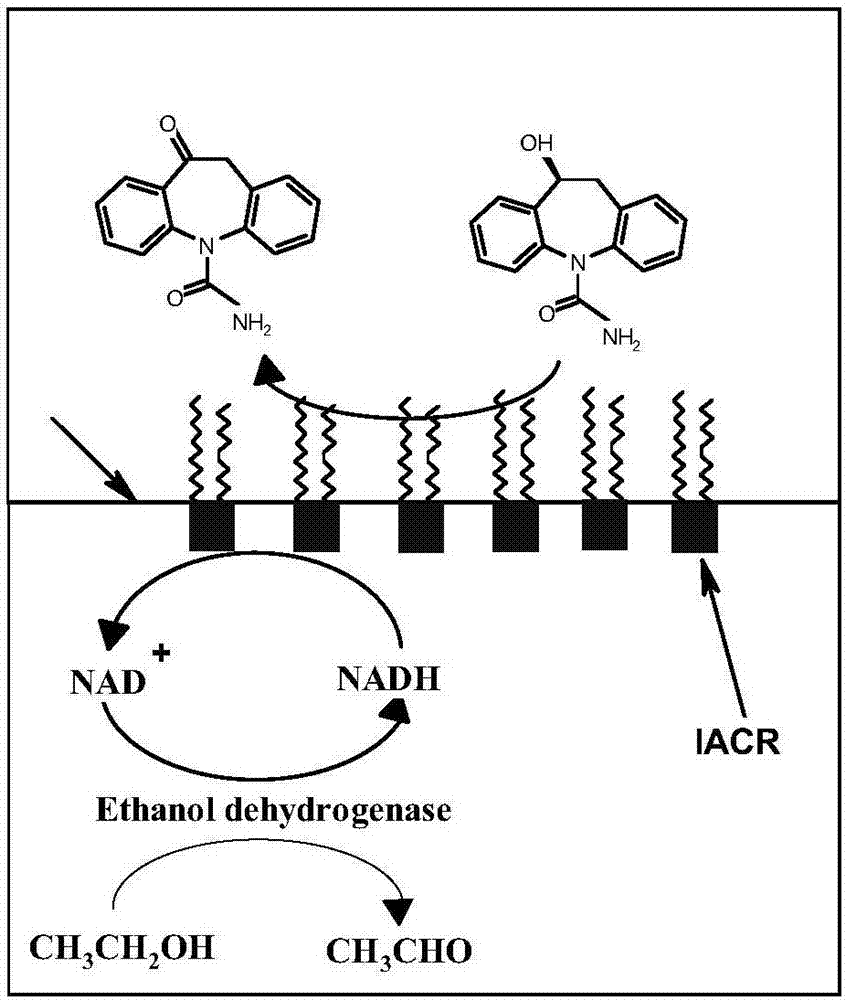
[0030] Add 5mg of carbonyl reductase enzyme solution (180U / mg) prepared in Example 1 into 6.25ml, pH 7.0, 0.02M Tris-HCl buffer solution to prepare 0.8g / L enzyme solution, then add 3.75g / L poly Styrene (Mw 10000Da) toluene solution 10ml was reacted for 1 hour in the dark at a shaker speed of 80r / min at 30°C. After the reaction is completed, centrifuge at 1200r / min for 10min, and the interfacial self-assembling enzyme is distributed on the two-phase interface of water and organic phase (that is, the upper layer is toluene, the middle is the interfacial self-assembling enzyme, and the lower layer is Tris-HCl buffer solution). Remove the buffer and organic solvent, wash the two-phase interface with toluene and pH 7.0, 0.02M Tris-HCl buffer solution successively for 3 times, remove free carbonyl reductase and poly...
Embodiment 3
[0040] (1) Preparation of interfacial self-assembly enzyme: the method is the same as in Example 1.
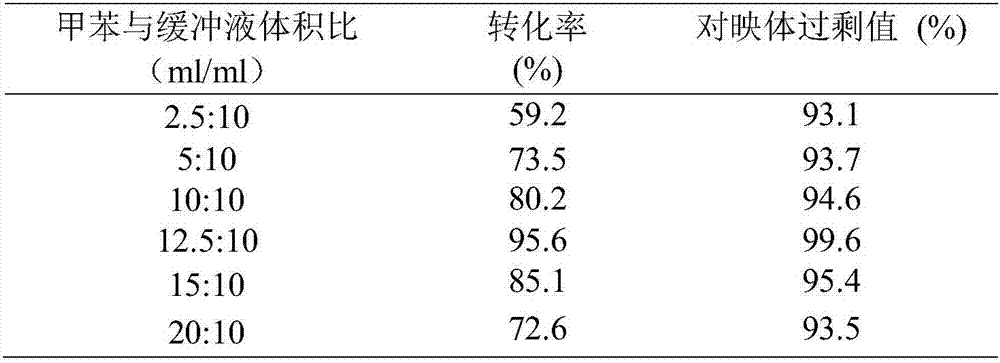
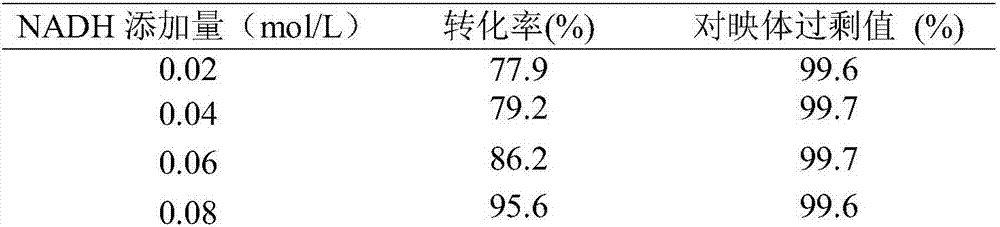
[0041] (2) Asymmetric reduction of oxcarbazepine: NADH with a buffer volume of 0.02, 0.04, 0.06, 0.08 and 0.10 mol / L was added to five 100ml conical flasks, Add 0.2mg alcohol dehydrogenase (enzyme activity 300U / mg), 10ml Tris-HCl (0.02mol / L, pH7.0) buffer solution, 0.6ml ethanol and the interfacial self-assembled carbonyl prepared in step (1) into the bottle respectively 2 mg (180 U / mg) of reductase, 12.5 ml of toluene, and a buffer volume concentration of 3.97 mmol / L oxcarbazepine were used for the biotransformation reaction. React at 30°C for 6h in a shaker at 80r / min. After the reaction, the reaction solution was centrifuged at 10,000 r / min for 10 min, and the toluene phase and the Tris-HCl buffer phase could be well separated. An organic phase containing the substrates oxcarbazepine and S-licarbazepine can be obtained after centrifugation. The Tris-HCl buffer solution o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com